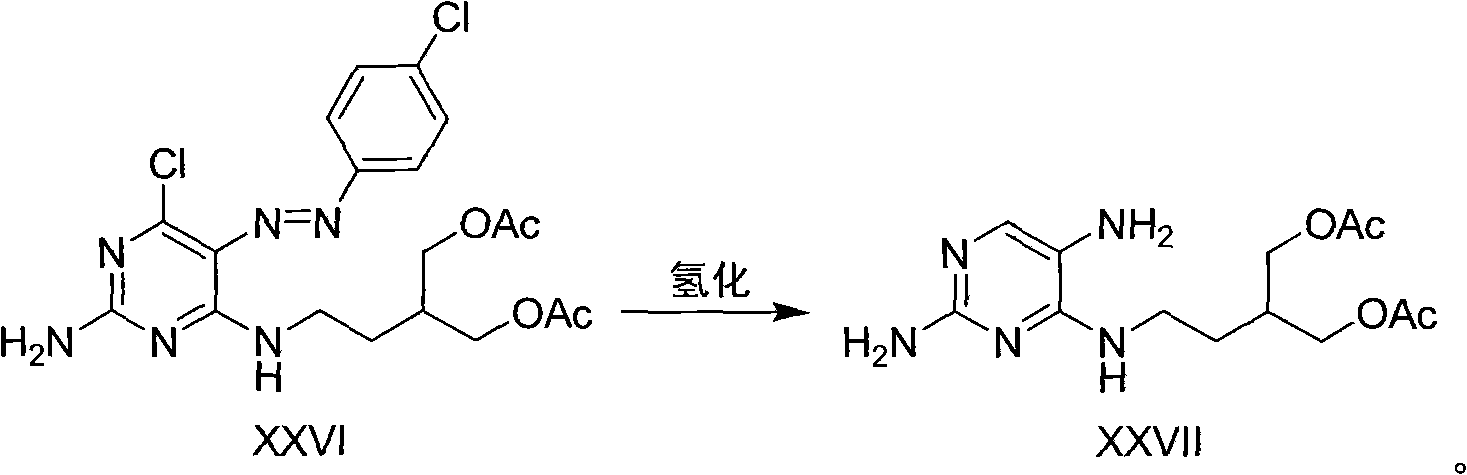
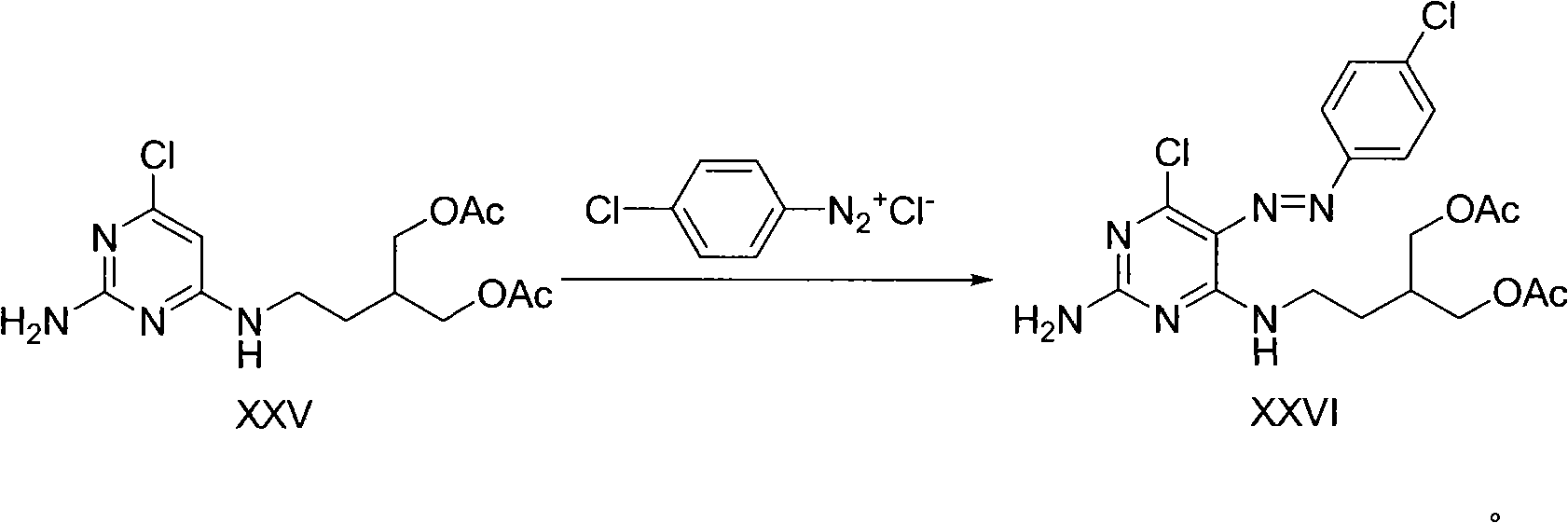
Method for synthesizing famciclovir
A famciclovir and amino technology, applied in organic chemistry, antiviral agents, etc., can solve the problem of high cost, achieve the effect of simple control, mild experimental conditions, and easy access to synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. 2-Amino-6-chloro-4-(3-hydroxymethyl-4-hydroxyl)-1-butyramine
[0052] In a 2000ml reaction bottle, dissolve 82 grams of 2-amino-4,6-dichloropyrimidine and 50 grams of 2-hydroxymethyl-4-amino-1-butanol in 1000ml of n-butanol, add potassium carbonate, and reflux for 5 Hour. The reaction solution was cooled, filtered, concentrated, dissolved in distilled water, and the aqueous phase was sequentially extracted with dichloromethane and n-butanol, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain light yellow oil or milky white solid. 1 H NMR (400MHz, d 6 -DMSO) δ6.94 (broad, 1H), 6.22 (s, 2H), 5.73 (s, 1H), 4.31 (t, J=4.6Hz, 2H), 3.45-3.43 (m, 4H), 3.24 (m , 2H), 1.49-1.55(m, 1H), 1.44-1.49(m, 2H); 13 C NMR (100MHz, d 6 -DMSO) δ164.5, 163.4, 157.6, 93.2, 62.0, 41.3, 38.7, 28.2; ESI-MS: 247 (M + +1); IR(film, cm -1 ): 3435, 3285, 3158, 1647, 1584, 1486; UV: 212.8nm.
Embodiment 2
[0054] 2. 2-Amino-6-chloro-4-(3-acetoxymethyl-4-acetoxy)-1-butyraminopyrimidine
[0055] Dissolve 5 grams of the crude product 2-amino-6-chloro-4-(3-hydroxymethyl-4-hydroxy)-1-butyraminopyridine in 50 ml of dichloromethane, add 8 ml of triethylamine at 0-10 °C, N, N-Dimethylaminopyridine and 4ml acetic anhydride, after reacting for 2-5 hours, the organic phase was washed with ice water, acetic acid aqueous solution and saturated sodium bicarbonate solution successively, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain Pale yellow solid 2-amino-6-chloro-4-(3-acetoxymethyl-4-acetoxy)-1-butyraminopyrimidine (6.67 g, yield: 99%). The solid was recrystallized from ethyl acetate, petroleum ether, dichloromethane, etc. 1 H NMR (400MHz, CDCl 3 )δ5.79(s, 1H), 5.21(s, 2H), 4.09-4.16(m, 4H), 3.51(broad), 2.10(s, 7H), 1.67(dt, J=7, 14Hz, 2H) ; 13 C NMR (100MHz, CDCl 3 )δ171.1, 164.1, 162.5, 93.0, 63.9, 38.7, 35.1, 28.2, 20.9, 20.6; ESI-MS: 331 (M + +1); IR...
Embodiment 3
[0057] 3. 2-Amino-6-chloro-5-p-chloroanilinodiazo-4-(3-acetoxymethyl-4-acetoxy)-1-butyraminopyrimidine
[0058] Dissolve 3 g of p-chloroaniline and 2 g of sodium nitrite in 20 ml of water, and react under the action of 3N hydrochloric acid at 0-10°C for 0.5-2 hours to prepare p-chloroaniline diazonium hydrochloride. The prepared diazonium salt was added to 6.7 g of 2-amino-6-chloro-4-(3-acetoxymethyl-4-acetoxy)-1-butyramine in a buffered solution of sodium acetate / acetic acid , reacted at 10-40°C for 0.5-5 hours, a large number of yellow solids precipitated out of the solution, filtered the yellow solids, and recrystallized from methanol to obtain 8.12 grams (yield: 86%) of 2-amino-6-chloro-5-p-chloroaniline Diazo-4-(3-acetoxymethyl-4-acetoxy)-1-butyraminopyrimidine. 1 H NMR (400MHz, CDCl 3 )δ10.32(m, 1H), 7.76(d, J=4.9Hz, 2H), 7.45(d, J=4.8Hz, 2H), 5.56(s, 2H), 4.26(dd, J=5.1, 11Hz , 2H), 4.18(dd, J=6.2, 11Hz, 2H), 3.68(dt, J=6.7, 13.6Hz, 2H), 2.14-2.17(m, 1H), 2.11(s, 6H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com