Famciclovir dispersible tablet and preparation method thereof
A technology of famciclovir and dispersible tablets, which is applied in the field of famciclovir dispersible tablets and its preparation, can solve the problems of low bioavailability and slow onset of action, and achieve the effect of improving clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
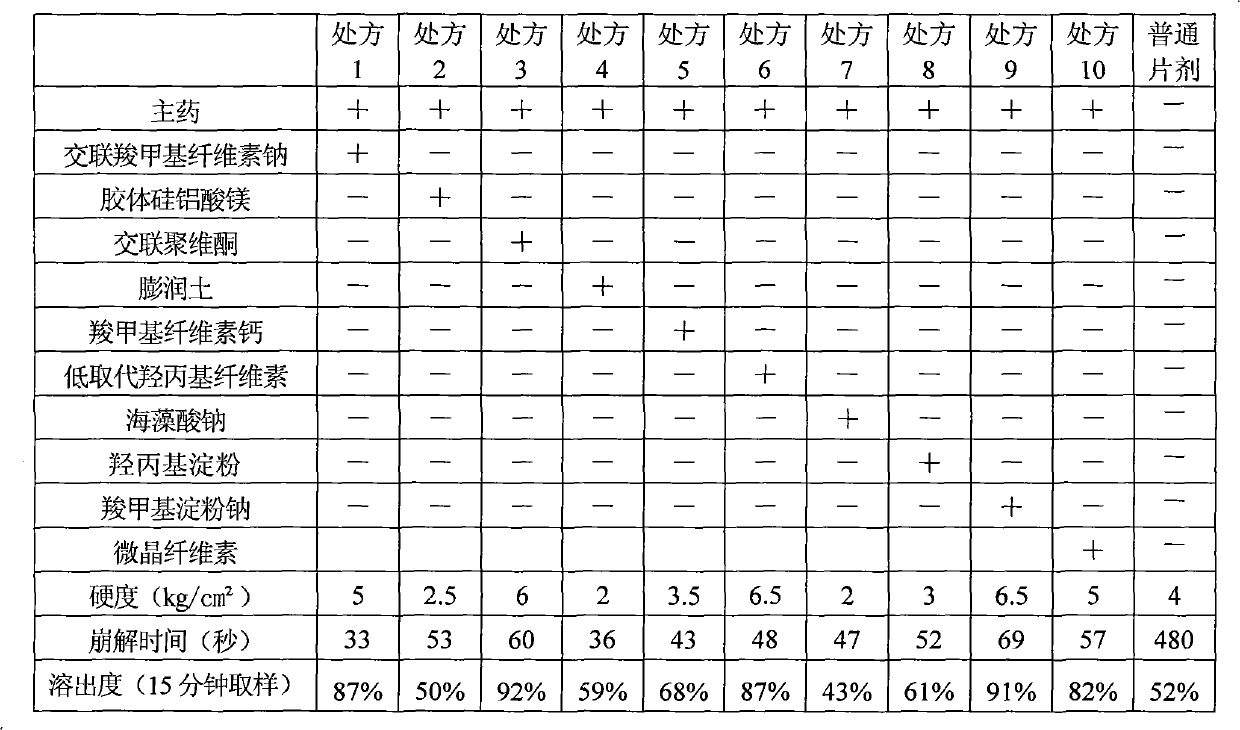
[0268] prescription:
[0269] Amount of raw materials used (g)
[0270] Famciclovir 250
[0271] Microcrystalline Cellulose 200
[0272] Sodium carboxymethyl starch 78
[0273] Crospovidone 60 (extra)
[0274] Magnesium Stearate 2.4(1.0%)
[0275] 8% PVPK30 ethanol solution appropriate amount
[0276]
[0277] Makes 1000 pieces
[0278] Preparation Process:
[0279] Accurately weigh the famciclovir raw material, pass through an 80-mesh sieve, add an appropriate amount of absolute ethanol as a binder, stir evenly to prepare a soft material, granulate with a 16-mesh sieve, and dry at 60°C to obtain famciclovir granules for future use.
[0280] Mix microcrystalline cellulose and 30g of sodium carboxymethyl starch evenly, pass through a 80-mesh sieve, add an appropriate amount of 8% PVPK30 ethanol solution as a binder, stir evenly to prepare a soft material, granulate with a 16-mesh sieve, and heat at 60°C Dry to obtain excipient granul...
Embodiment 2
[0282] prescription:
[0283] Amount of raw materials used (g)
[0284] Famciclovir 250
[0285] Pregelatinized starch 300
[0286] Croscarmellose sodium 72 (28g internally, 44g externally)
[0287] Crospovidone 50 (extra)
[0288] Micronized silica gel 0.5%
[0289] 8% PVPK30 ethanol solution appropriate amount
[0290]
[0291] Makes 1000 pieces
[0292] Preparation Process:
[0293] Accurately weigh the famciclovir raw material, pass through a 100-mesh sieve, add an appropriate amount of absolute ethanol as a binder, stir evenly to prepare a soft material, granulate with a 16-mesh sieve, and dry at 60°C to obtain famciclovir granules for future use.
[0294] Mix the pregelatinized starch and croscarmellose sodium added internally, pass through a 100-mesh sieve, add an appropriate amount of 8% PVPK30 ethanol solution as a binder, stir evenly to prepare a soft material, granulate with a 16-mesh sieve, 60 Dry at ℃ to obtain excipient...
Embodiment 3
[0296] prescription:
[0297] Amount of raw materials used (g)
[0298] Famciclovir 250
[0299] Microcrystalline Cellulose 250
[0300] Sodium carboxymethyl starch 78 (30g internally, 48g externally)
[0301] Low-substituted hydroxypropyl cellulose 55 (extra)
[0302] Magnesium Stearate 1.0%
[0303] 8% PVPK30 ethanol solution appropriate amount
[0304]
[0305] Makes 1000 pieces
[0306] Preparation Process:
[0307] Accurately weigh the famciclovir raw material, pass through a 100-mesh sieve, add an appropriate amount of absolute ethanol as a binder, stir evenly to prepare a soft material, granulate with a 16-mesh sieve, and dry at 60°C to obtain famciclovir granules for future use.
[0308] Mix microcrystalline cellulose and internally added sodium carboxymethyl starch evenly, pass through a 100-mesh sieve, add an appropriate amount of 8% PVPK30 ethanol solution as a binder, stir evenly to prepare a soft material, granulate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com