Preparation of famciclovir and other purine derivatives
a technology of purine derivatives and famciclovir, which is applied in the field of purine derivative preparation of various 9substituted purine derivatives, can solve the problems of reducing yield and requiring a separation step to remove unwanted substances, low overall yield of famciclovir, and low yield of starting compounds 8 and 9 commercially available, etc., and achieves high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Famciclovir
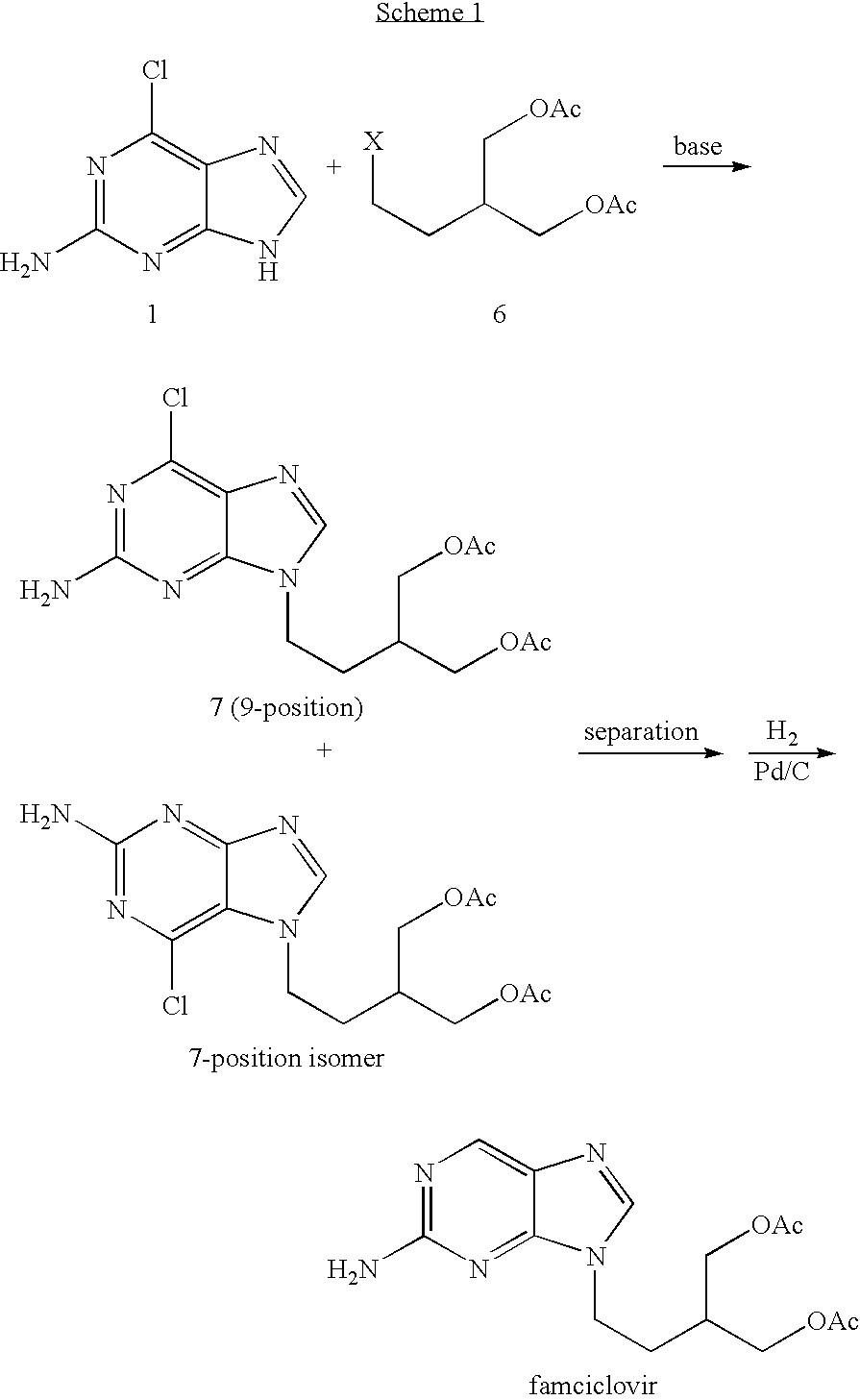
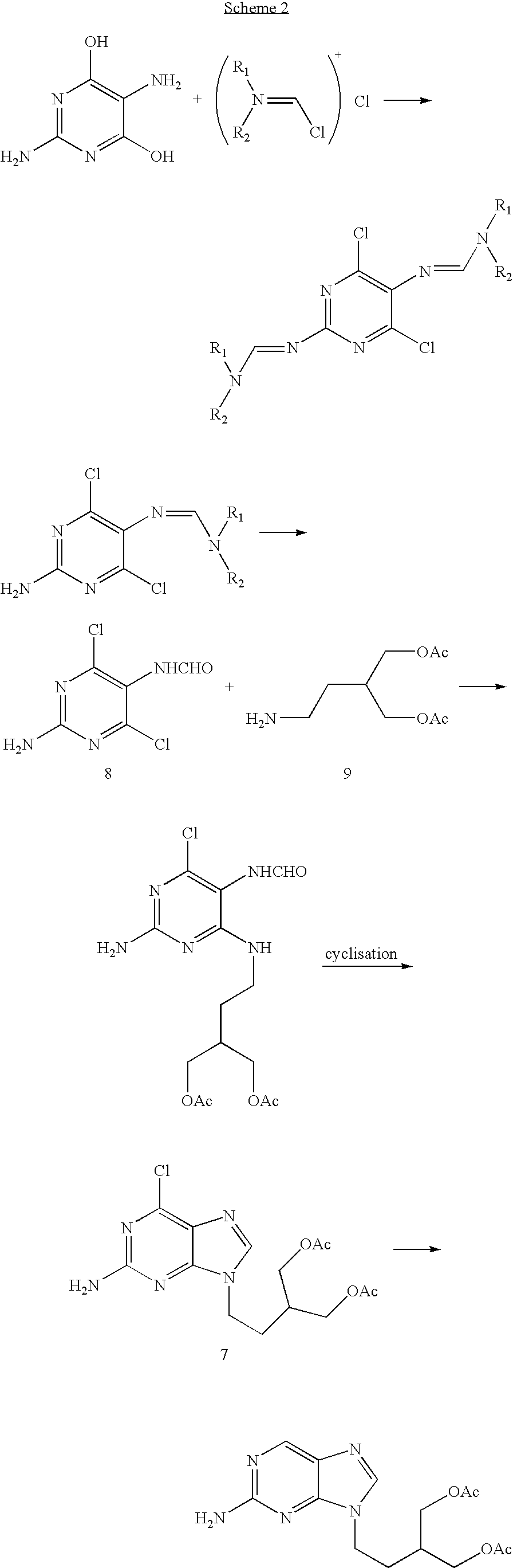
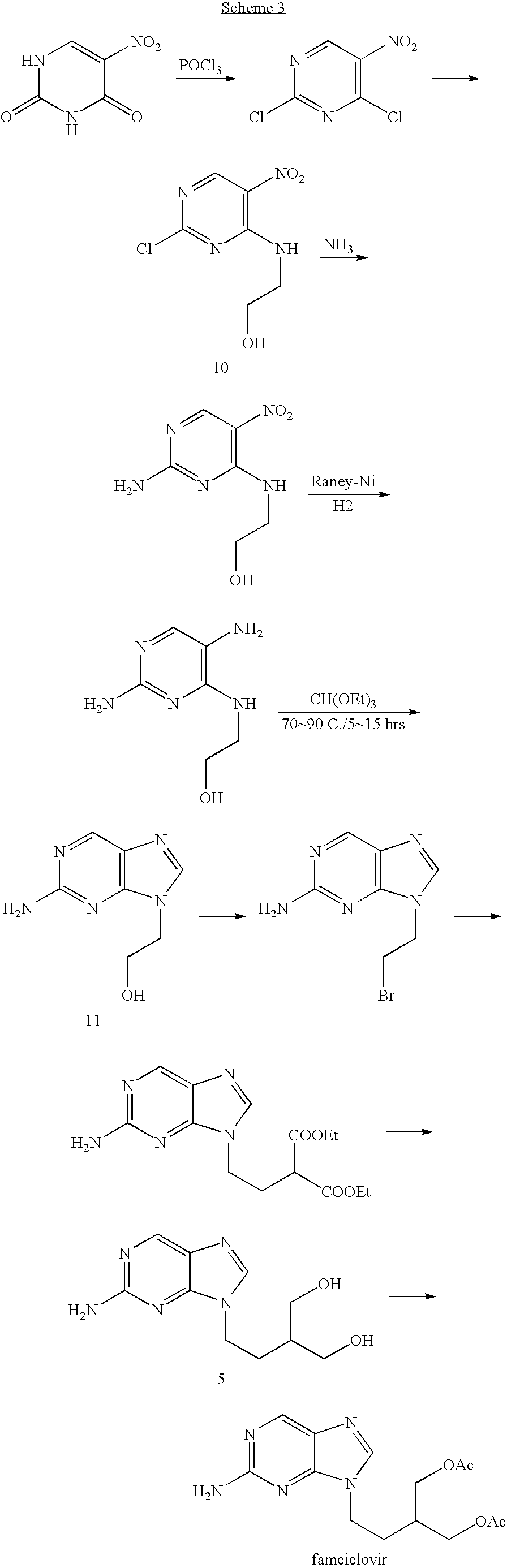
[0032] Famciclovir was prepared according to the following synthetic scheme:
[0033] The steps were as follows.
Step 1: Preparation of 2-amino-6-9-chloro-(2-bromoethyl)purine (2)
[0034] A mixture of 2-amino-6-chloropurine (33.9 g, 0.2 mol), potassium carbonate (69 g, 0.5 mol) and DMF (340 ml) were placed in a 1 L 3-neck flask, and heated at 60-65° C. for 1 hour. Then the 1,2-dibromoethane (112.8 g, 0.6 mol) was added and the resulting mixture was refluxed for 24 hours. The reaction mixture was then cooled and filtered. The filtrate solution was concentrated by distillation at reduced pressure. The residue was dissolved with methanol (170 ml) and cooled down to 0˜5° C. The titled compound 2 was obtained in crystalline form (50.3 g, yield 91%).
Step 2: Preparation of diethyl 2-[2-(2-amino-6-chloro-9H-purine-9-yl)-ethyl]-1,3-malonate (3)
[0035] To a dried 1 L reaction flask were added sequentially the DMF (380 g), anhydrous K2CO3 (55 g, 0.04 mol), intermed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com