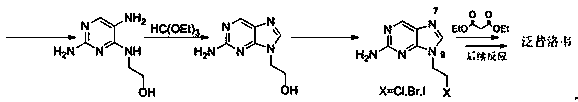
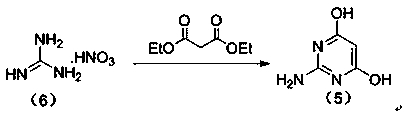Preparation method of famciclovir
A technology of famciclovir and amino, which is applied in the field of medicine, can solve the problems of poor selectivity of N-alkylation reaction, potential safety hazards in the use of diazonium salts, and high production costs, and achieve low prices, improved atom economy, and low production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The first step, the synthesis of 2-amino-4,6-pyrimidinediol
[0055] Control the temperature at 5°C, put 30.52g (0.25mol) of guanidine nitrate and 100ml of methanol into a 500ml four-necked bottle, start stirring, and slowly drop in 250ml of 2.5M sodium methoxide methanol solution after all the solids are dissolved, and keep stirring for 0.5h; Subsequently, 41.64 g (0.26 mol) of diethyl malonate was slowly dropped into the above-mentioned reaction flask, and after the drop was completed, the temperature was raised to reflux for 6 hours. After the reaction was completed, it was concentrated under reduced pressure to obtain an off-white solid. Add 60ml of drinking water to the reaction bottle to dissolve the solid, then adjust the pH value of the system to 6 with 10% dilute hydrochloric acid solution, at this time a large amount of solid precipitates; ℃ blast drying to constant weight to obtain 29.74 g of off-white product, the yield of this step is 93.6%, and the HPLC p...
Embodiment 2
[0057] The first step, the synthesis of 2-amino-4,6-pyrimidinediol
[0058] Control the temperature at 5°C, put 30.52g (0.25mol) of guanidine nitrate and 100ml of absolute ethanol into a 500ml four-necked bottle, start stirring, and slowly drop in 250ml of 2.5M sodium methoxide ethanol solution after all the solids are dissolved, keep stirring for 0.5 h; then slowly drop 41.64 g (0.26 mol) of diethyl malonate into the above-mentioned reaction flask, after the drop is complete, raise the temperature to 65° C. for 6 h. After the reaction was completed, it was concentrated under reduced pressure to obtain an off-white solid. Add 60ml of drinking water to the reaction bottle to dissolve the solid, then adjust the pH value of the system to 6 with 10% dilute hydrochloric acid solution, at this time a large amount of solid precipitates; ℃ blast drying to constant weight, to obtain 30.56 g of off-white product, the yield of this step is 96.10%, and the HPLC purity is 99.7%.
Embodiment 3
[0060] The first step, the synthesis of 2-amino-4,6-pyrimidinediol
[0061] Control the temperature at 5°C, put 30.52g (0.25mol) of guanidine nitrate and 100ml of absolute ethanol into a 500ml four-neck bottle, start stirring, and slowly drop in 250ml of 2.5M sodium ethoxide ethanol solution after all the solids are dissolved, keep stirring for 0.5 h; then slowly drop 41.64 g (0.26 mol) of diethyl malonate into the above reaction flask, after the drop is complete, heat up to reflux for 4 hours. After the reaction was completed, it was concentrated under reduced pressure to obtain an off-white solid. Add 60ml of drinking water to the reaction bottle to dissolve the solid, then adjust the pH value of the system to 6 with 10% dilute hydrochloric acid solution, at this time a large amount of solid precipitates; ℃ blast drying to constant weight to obtain 28.78 g of off-white product, the yield of this step is 90.59%, and the HPLC purity is 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com