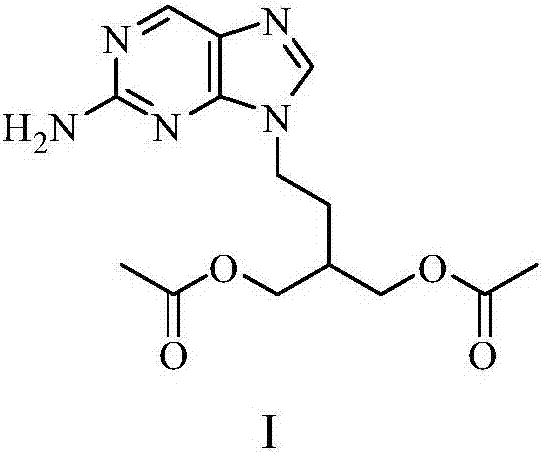
Famciclovir sustained-release granules and production method thereof
A technology of sustained-release granules and famciclovir, which is applied in the field of medicine, can solve the problems of unfavorable patient compliance, does not solve the problem of frequent taking of famciclovir, etc., and achieves the effect of reducing the frequency of taking medicine, improving compliance and good taste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
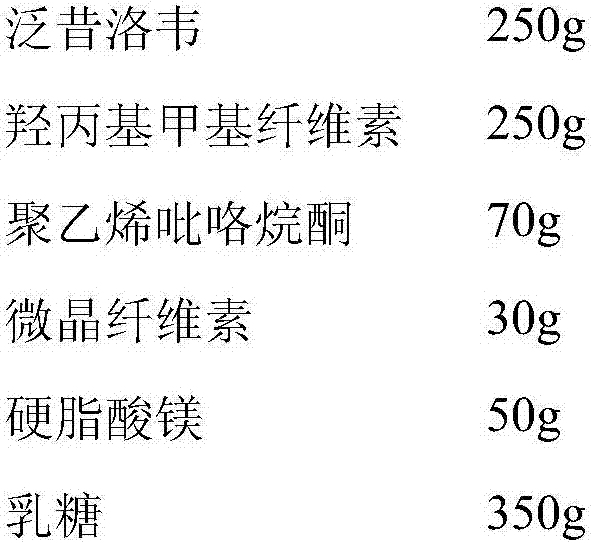
[0034] The preparation of embodiment 1 famciclovir sustained-release granules
[0035]
[0036] The raw and auxiliary materials are respectively passed through a 100-mesh sieve, and the raw materials and hydroxypropyl methylcellulose are weighed, and the raw and auxiliary materials are preliminarily mixed by an equal-volume increasing method, and then the prescribed amount of polyvinylpyrrolidone and microcrystalline cellulose are added, and an appropriate amount of 95% ethanol, made of soft material, granulated through a 16-mesh sieve, dried at 50°C for 2 hours, then granulated through a 16-mesh sieve, added with 5% magnesium stearate and 35% lactose and mixed evenly.
Embodiment 2
[0037] The preparation of embodiment 2 famciclovir sustained-release granules comparative example 1
[0038]
[0039]
[0040] The raw and auxiliary materials are respectively passed through a 100-mesh sieve, the bulk drug and hydroxypropyl methylcellulose are weighed, the raw and auxiliary materials are preliminarily mixed by an equal increase method, then the prescribed amount of microcrystalline cellulose is added, and an appropriate amount of 95% ethanol is added, Make soft material, pass through a 16-mesh sieve to granulate, dry at 50°C for 2 hours, pass through a 16-mesh sieve for granulation, add magnesium stearate and lactose and mix well.
Embodiment 3
[0041] The preparation of embodiment 3 famciclovir sustained-release granules comparative example 2
[0042]
[0043] The raw and auxiliary materials were respectively passed through a 100-mesh sieve, and the bulk drug was weighed, and the prescribed amount of polyvinylpyrrolidone and microcrystalline cellulose was added, and an appropriate amount of 95% ethanol was added to make a soft material, passed through a 16-mesh sieve for granulation, dried at 50°C for 2 hours, and then Sieve through a 16-mesh sieve for granulation, add magnesium stearate and lactose and mix well.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com