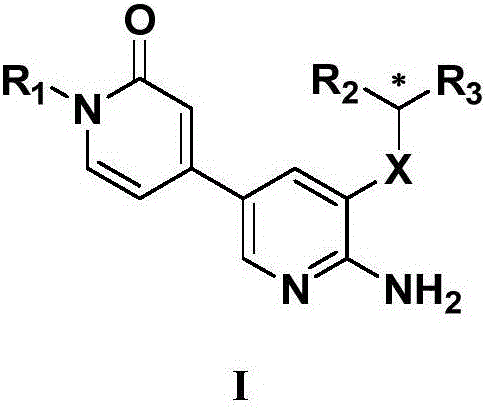
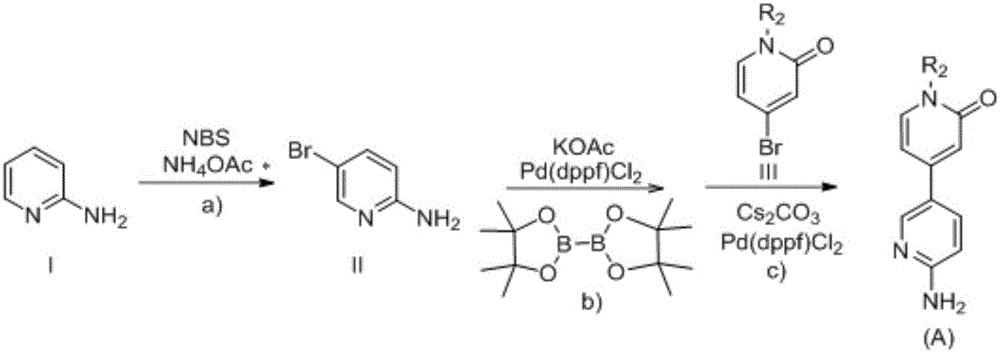
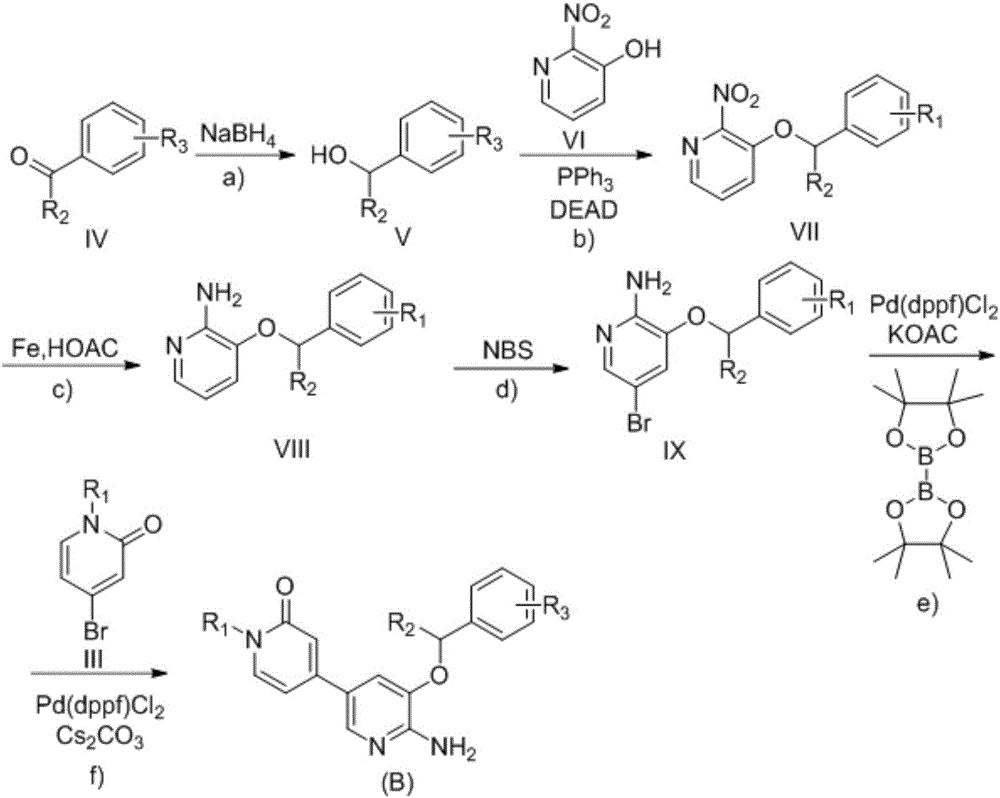
2-aminopyridine derivative containing 2-pyridone ring side chain, preparation and application
A technology of aminopyridine and pyridone rings, applied in the field of 2-aminopyridine derivatives, can solve the problems of drug resistance and affecting the binding affinity of ALK tyrosine kinase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: 6-amino-[3,4'-bipyridine]-2'(1'H)-one (compound 1):
[0083]
[0084] step:
[0085] 5-Bromo-2-aminopyridine (10.0 mmol) was dissolved in 20 mL of anhydrous dioxane with pinacol diboronate (11.0 mmol) and potassium acetate (15.0 mmol), and then Pd(dppf)Cl was added 2 (0.5mmol), replaced with nitrogen, heated at 80°C for 8h, monitored the reaction by LC-MS until the disappearance of the raw material. After the reaction is naturally cooled, without separation, directly add cesium carbonate (15.0mmol) and Pd(dppf)Cl to the reaction system 2 (0.5mmol) and 4-bromopyridin-2(1H)-one (11.0mmol), then add 0.5mL water, and stir overnight at 110°C. After the reaction was completed, the reaction system was filtered with diatomaceous earth, the filtrate was spin-dried, and then extracted with DCM. Combine the organic phases, then anhydrous Na 2 SO 4 Drying, filtration, rotary evaporation, the crude product was separated by silica gel column chromatography (DCM:MeO...
Embodiment 2
[0086] Example 2: 6-Amino-1'-methyl-[3,4'-bipyridine]-2'(1'H)-one
[0087] Referring to the method of Example 1, only the raw material 4-bromopyridin-2(1H)-one was replaced with 4-bromo-1-methyl-2(1H)-one, after the reaction was completed, the crude product was obtained by concentrating under reduced pressure to dryness , purified by column chromatography (DCM / MeOH=10:1) to obtain a yellow solid with a yield of 68%.
[0088] m.p.233-236°C; 1 H NMR (500MHz, DMSO) δ8.32(d, J=2.3Hz, 1H), 7.74(dd, J=8.7, 2.6Hz, 1H), 7.66(d, J=7.1Hz, 1H), 6.55(d ,J=1.9Hz,1H),6.52(dd,J=7.1,2.1Hz,1H),6.50–6.45(m,1H),6.36(s,2H),3.39(s,3H); HRMS(ESI) :m / z calcd for C 11 h 11 N 3 O[M+H] + :202.0975,Found:202.0976.m.p.>250℃;
Embodiment 3
[0089] Example 3: 6-Amino-1'-benzyl-[3,4'-bipyridine]-2'(1'H)-one
[0090] Referring to the method of Example 1, only the raw material 4-bromopyridin-2(1H)-one was replaced with 1-benzyl-4-bromo-2(1H)-one, and after the reaction was completed, it was concentrated to dryness under reduced pressure to obtain the crude product , purified by column chromatography (DCM / MeOH=10:1) to give a white solid with a yield of 60%.
[0091] m.p.207-209°C; 1 H NMR (500MHz, DMSO) δ8.33 (s, 1H), 7.75 (dd, J = 10.2, 4.5Hz, 2H), 7.38–7.20 (m, 5H), 6.65–6.54 (m, 2H), 6.48 ( d,J=8.7Hz,1H),6.38(s,2H),5.08(s,2H); HRMS(ESI):m / z calcd for C 17 h 15 N 3 O[M+H] + :278.1288,Found:278.1288.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com