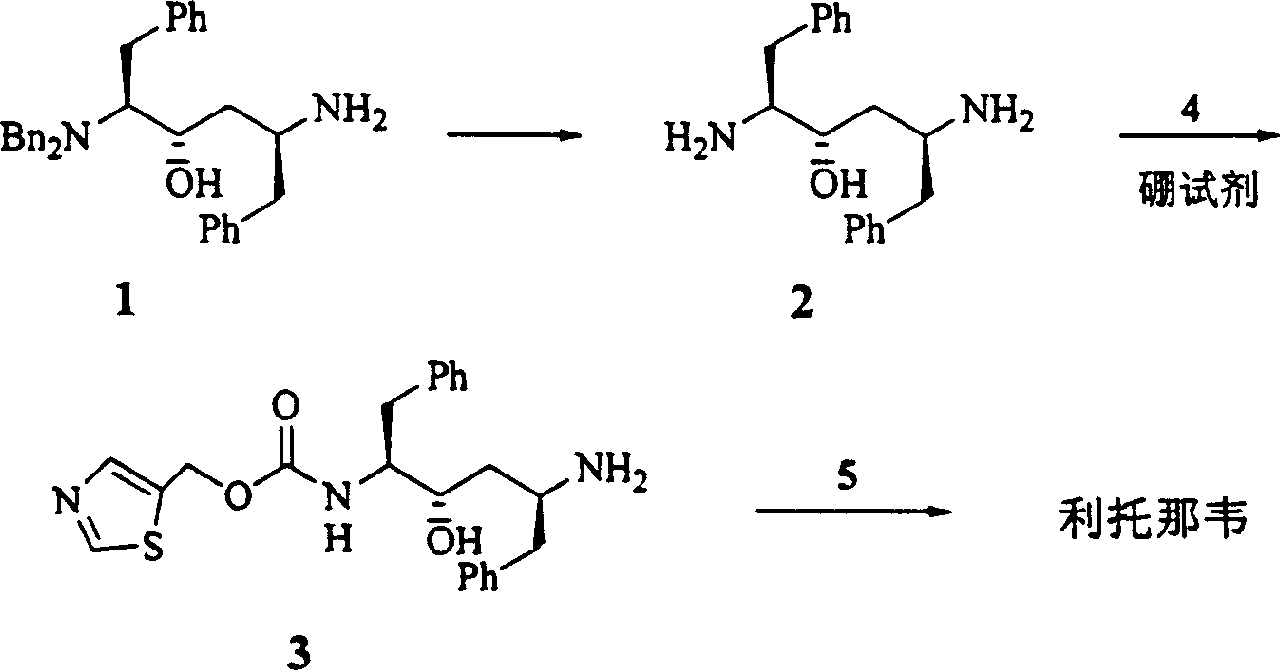
Process for synthesizing ritonavir
A technology of ritonavir and amino group, which is applied in the field of synthesizing ritonavir, can solve the problems affecting thiazole methylamine group, difficult control of hydrogenolysis debenzyl group selectivity, selectivity problems, etc., to achieve reaction specificity Strong, improved atomic utilization, and cost-reducing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] Below by embodiment the present invention will be further described.
[0023] Step 1 Benzylaminoalcohol 1 (696mg, 1.5mmol) was dissolved in anhydrous dichloromethane (30ml). Cool to -20°C. A solution of N-carboxylic acid internal anhydride 8 in dichloromethane (2.25ml, 2.25mmol) was slowly added dropwise. After the addition was complete, 30 ml of a dichloromethane solution of triethylamine (0.27 ml, 1.92 mmol) was slowly added dropwise over 10 min. React at -13~-17°C for 2 hours. Adjust the pH to 4 with concentrated hydrochloric acid. Adjust the pH to 8-9 with saturated sodium bicarbonate solution. wash. Dry over anhydrous sodium sulfate. Concentrate under reduced pressure to obtain 991 mg of valyl ampicillin.
[0024] 1 H NMR (CDCl 3 , 500MHz) δ7.05-7.35(m, 20H), 4.12(m, 1H), 3.90(d, J=13.3Hz, 2H), 3.57(dt, J=2.2×8.1Hz, 1H), 3.38(d , J=14.2Hz, 2H), 3.10(d, J=4.1Hz, 1H), 3.04(dd, J=14.2×5.8Hz, 1H), 2.62-2.82(m, 4H), 2.15(m, 1H) , 1.57(m, 2H), 1.53(m, 2H), 1.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com