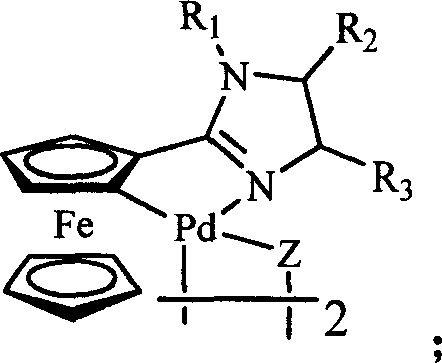
Ferrocenyl imidazoliny palladium compound, its preparation method and its uses in catalytic synthesis of coupling product
A ferrocenyl imidazoline, compound technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, chemical instrument and method, etc., can solve the problem that cannot be widely used in industrial production, chemical Uncertain selectivity, large amount of catalyst, etc., to achieve the effect of insensitivity to water or air, good thermal stability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of 1-benzyl-2-ferrocenyl-4,5-dihydroimidazoline ring palladium chlorine bridge dimer: preparation of ferrocenyl imidic acid methyl ester salt according to the method reported in the literature Under nitrogen protection, methyl ferrocenyl imidate hydrochloride (280mg, 1mmol) and N-benzylethylenediamine (150mg, 1mmol) were stirred in 25mL of anhydrous methanol at 20°C for 0.5 hours, then Reflux for 10 hours. After the reaction was completed, methanol was removed by rotary evaporation, 30 mL of dichloromethane was added, washed twice with saturated sodium bicarbonate solution, then washed twice with saturated brine, the organic phases were combined, anhydrous MgSO 4Dry, filter, concentrate by rotary evaporation, and use 10:1 dichloromethane / methanol as developing solvent for the residue, and separate by silica gel column chromatography to obtain 159 mg of pure orange-red solid as 1-benzyl-2-ferrocenyl-4, 5-dihydroimidazoline (R 1 = benzyl, R 2 = R...
Embodiment 2
[0049] Example 2: Preparation of 1-benzyl-2-ferrocenyl-4,5-dihydroimidazoline ring palladium bromide bridge dimer: prepare 1-benzyl-2-dimer as in Example 1 Ferrocenyl-4,5-dihydroimidazoline, 5ml 0.1M Li 2 PdBr 4 Methanol solution and 68 mg NaOAc 3H 2 O was added to 1-benzyl-2-ferrocenyl-4,5-dihydroimidazoline (R 1 = benzyl, R 2 = R 3 = R 4 = R 5 =H) (172mg, 0.5mmol) in methanol solution, stirred at room temperature for 24 hours, filtered off to form a brown solid, that is, 1-benzyl-2-ferrocenyl-4,5-dihydroimidazoline ring palladium Bromine Bridged Dimer.
Embodiment 3
[0050] Example 3: Preparation of 1-benzyl-2-ferrocenyl-4,5-dihydroimidazoline ring palladium iodine bridge dimer: prepare 1-benzyl-2-dimer as in Example 1 Ferrocenyl-4,5-dihydroimidazoline, 5ml 0.1M Li 2 PdI 4 Methanol solution and 68 mg NaOAc 3H 2 O was added to 1-benzyl-2-ferrocenyl-4,5-dihydroimidazoline (R 1 = benzyl, R 2 = R 3 = R 4 = R 5 =H) (172mg, 0.5mmol) in methanol solution, stirred at room temperature for 24 hours, filtered off to form a brown solid, that is, 1-benzyl-2-ferrocenyl-4,5-dihydroimidazoline ring palladium Iodine-bridged dimers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com