Polycarbonate material and preparation method thereof
A technology of polycarbonate and copolymerization reaction, applied in the field of polymers, can solve problems such as complex structure, and achieve the effect of simple preparation method and good structure control ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The embodiment of the invention discloses a preparation method of a polycarbonate material, comprising:
[0024] Copolymerize carbon dioxide and epoxy compounds containing long-chain alkane substituents under the action of a catalyst to obtain polycarbonate materials.
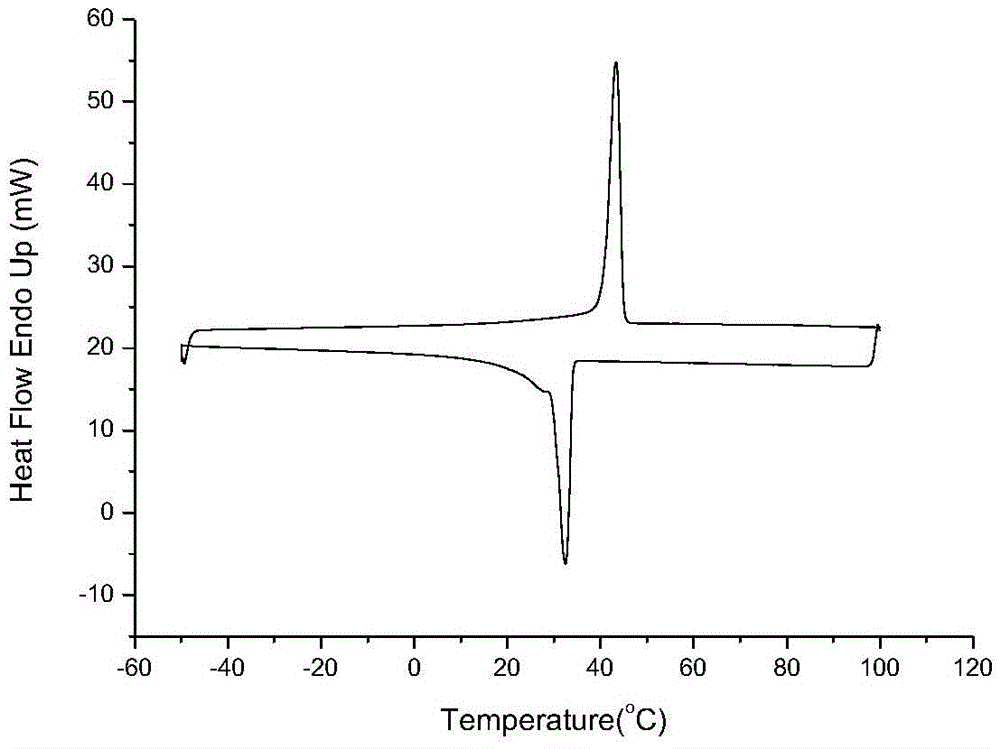
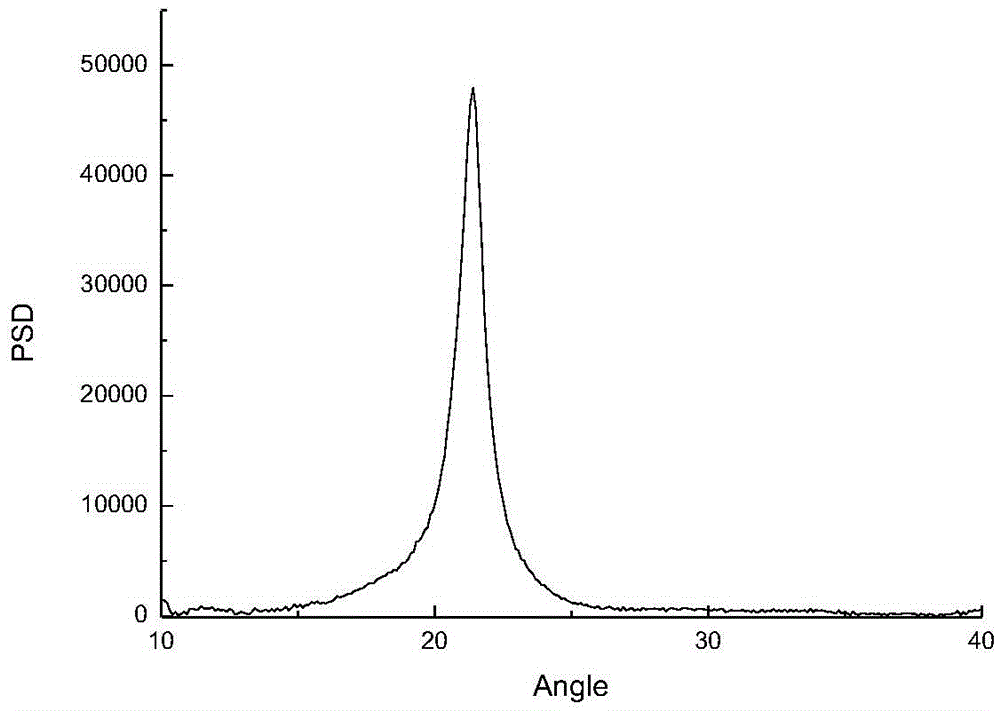
[0025] This application uses carbon dioxide and epoxy compounds containing long-chain alkane substituents as raw materials to synthesize polycarbonate materials under the action of catalysts. The polycarbonate material prepared by the present application has crystallinity, and with the continuous increase of the side chain length of the epoxy compound, the crystallization ability of the polymer is continuously enhanced.
[0026] In the process of preparing polycarbonate materials in the present application, the catalyst is selected from the first catalytic system of tetradentate Schiff base cobalt complex and cocatalyst, the second catalytic system of porphyrin cobalt and cocatalyst, and the second catal...
Embodiment 1 4
[0042] Embodiment 1 tetradentate Schiff base cobalt complex (SalanCo III Cl) Preparation of catalyst
[0043] Step a) Add 3,5-di-tert-butyl salicylaldehyde (34.85g, 0.1mol) and 200mL ethanol successively in a 500mL three-necked flask equipped with magnetic stirring, reflux condenser and constant pressure dropping funnel, and heat to 60 At about ℃, stir to dissolve it completely; dissolve 1,2-cyclohexanediamine (5.7g, 0.05mol) in 40mL ethanol, slowly add dropwise to the ethanol solution of salicylaldehyde, after the dropwise addition, heat to reflux for 6h , cooled to room temperature, and left overnight. The resulting mixture was filtered to obtain a yellow solid, which was then dried under vacuum at 40°C to obtain the Salen ligand.
[0044] Step b) Under argon protection, add Salen ligand (3.65 g, 6.67 mmol) and 25 mL of refined dichloromethane into a 250 mL three-neck flask equipped with a constant pressure dropping funnel and magnetic stirring, and stir to dissolve it. A...
Embodiment 2 4
[0048] The preparation of embodiment 2 tetraphenylporphyrin cobalt
[0049] Step a) Add 800mL of refined methylene chloride, benzaldehyde (2.2g, 13mmol) and pyrrole (0.93mL, 13mmol) successively to a 1L three-necked flask equipped with magnetic stirring and protected by argon, react at room temperature, and stir to make it complete dissolve. Trifluoroacetic acid (0.93 mL, 4 mmol) was slowly added dropwise to the above solution, and after the dropwise addition was completed, it was reacted for 1 h to obtain a reaction solution. Dichlorodicyanobenzoquinone (1.82 g, 8 mmol) was added to the reaction liquid, and the reaction was continued for 1 h, then filtered, and the solvent was spun off. The crude product was chromatographed on a neutral alumina column (eluent: petroleum ether / dichloromethane) to obtain a pure purple solid.
[0050] Step b) Under the protection of argon, add the product of the previous step (6.12g, 10mmol) into a dry 1L three-necked flask, add 500mL N, N-dim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com