Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Imidic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
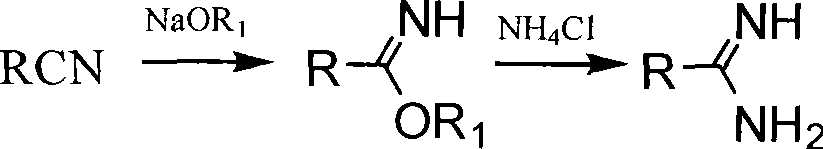
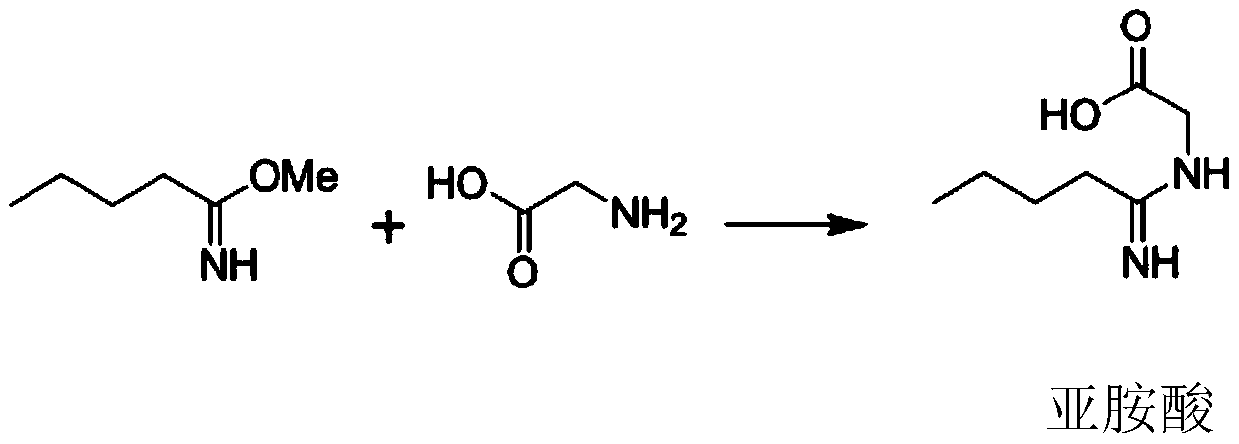
In chemistry, an imidic acid is any molecule that contains the -C(=NH)-OH functional group. It is the tautomer of an amide and the isomer of an oxime. The term "imino acid" is an obsolete term for this group that should not be used in this context because an imino acid has a different technical meaning.
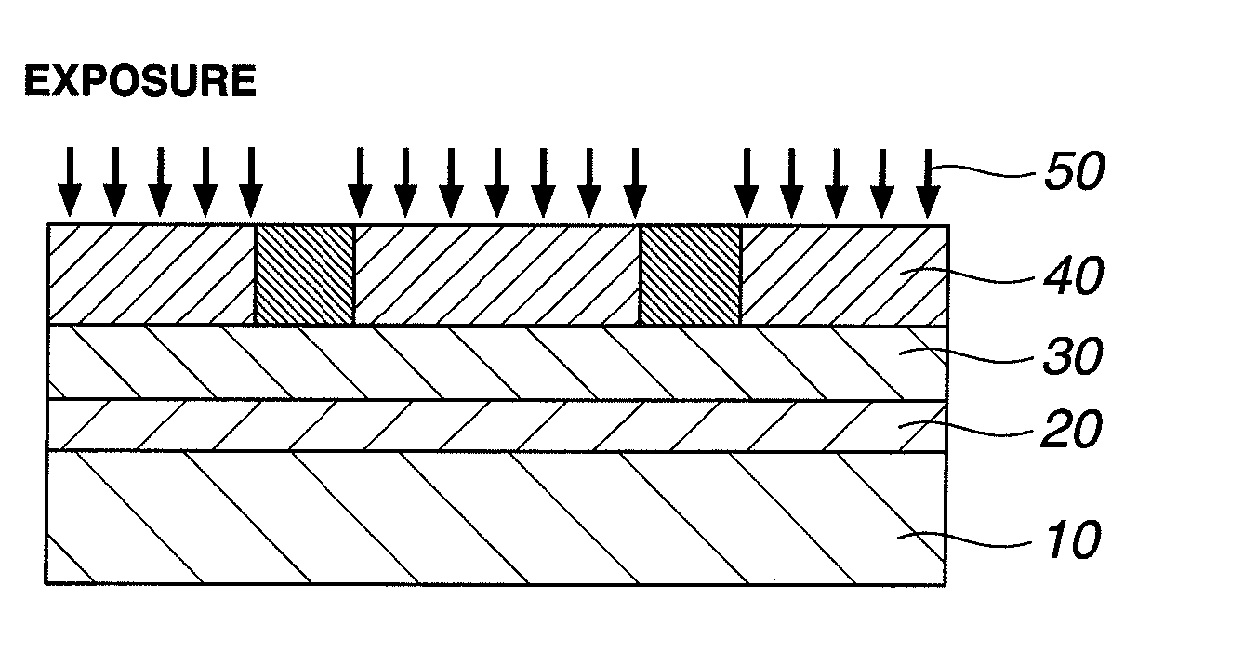
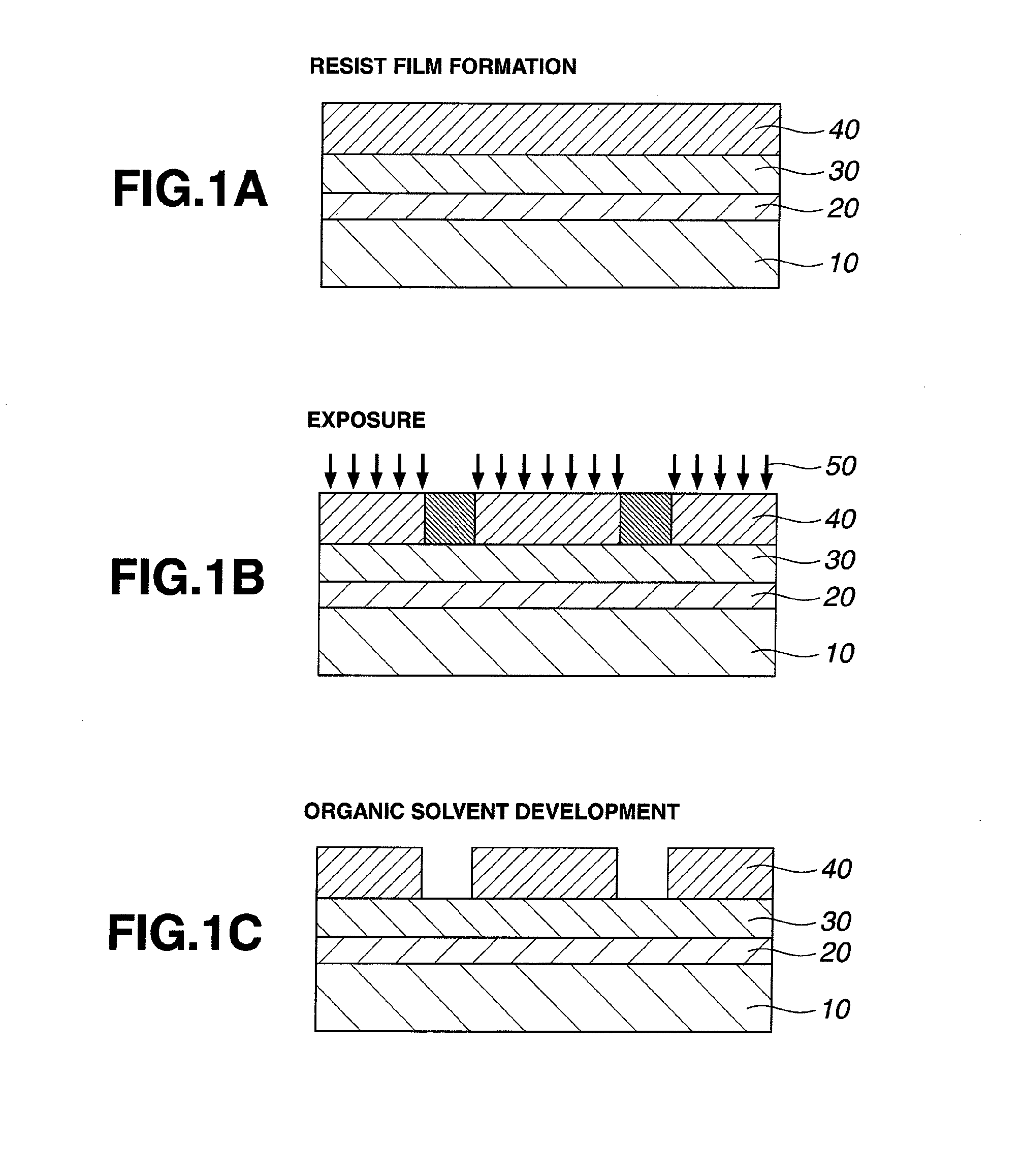
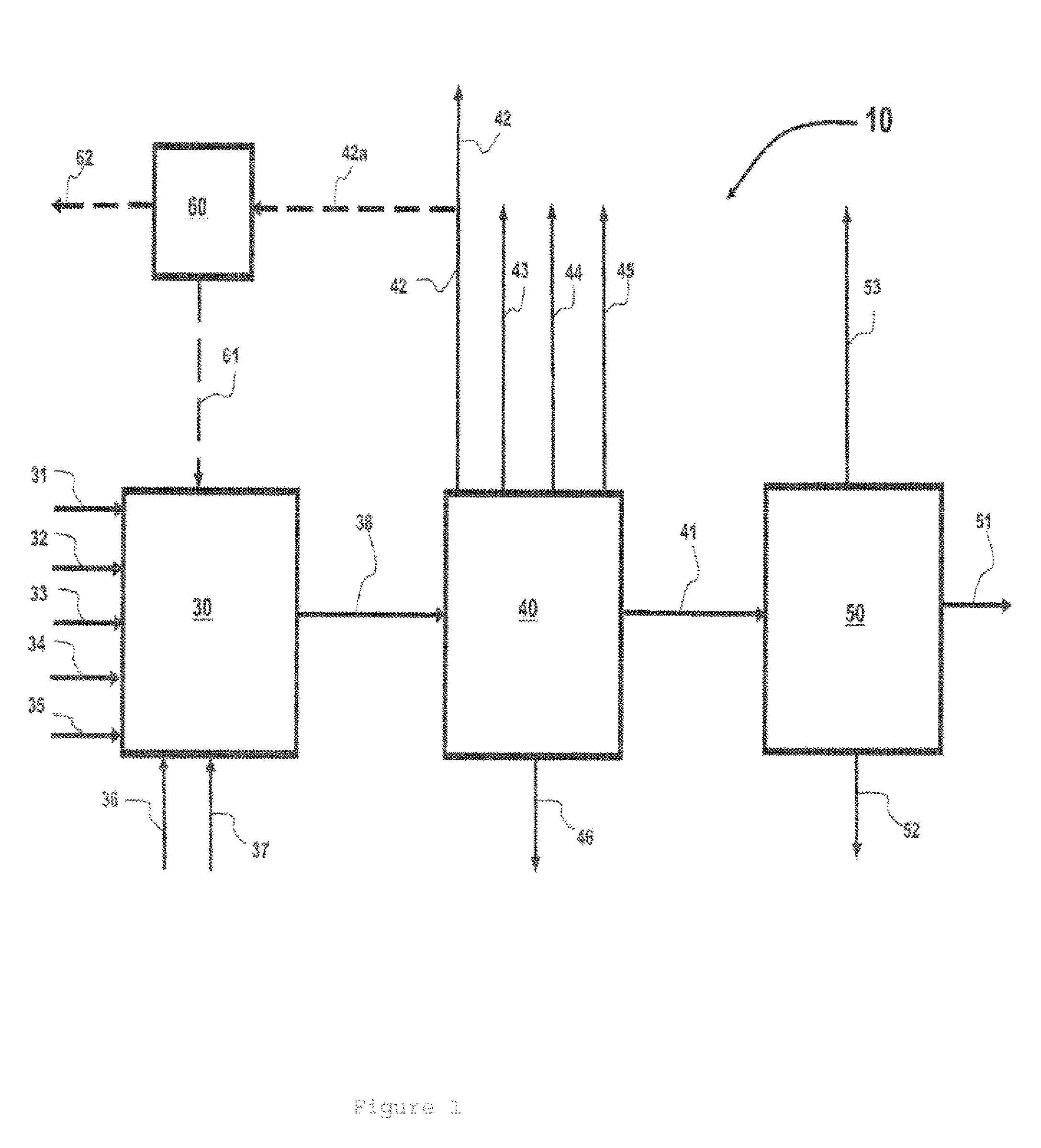
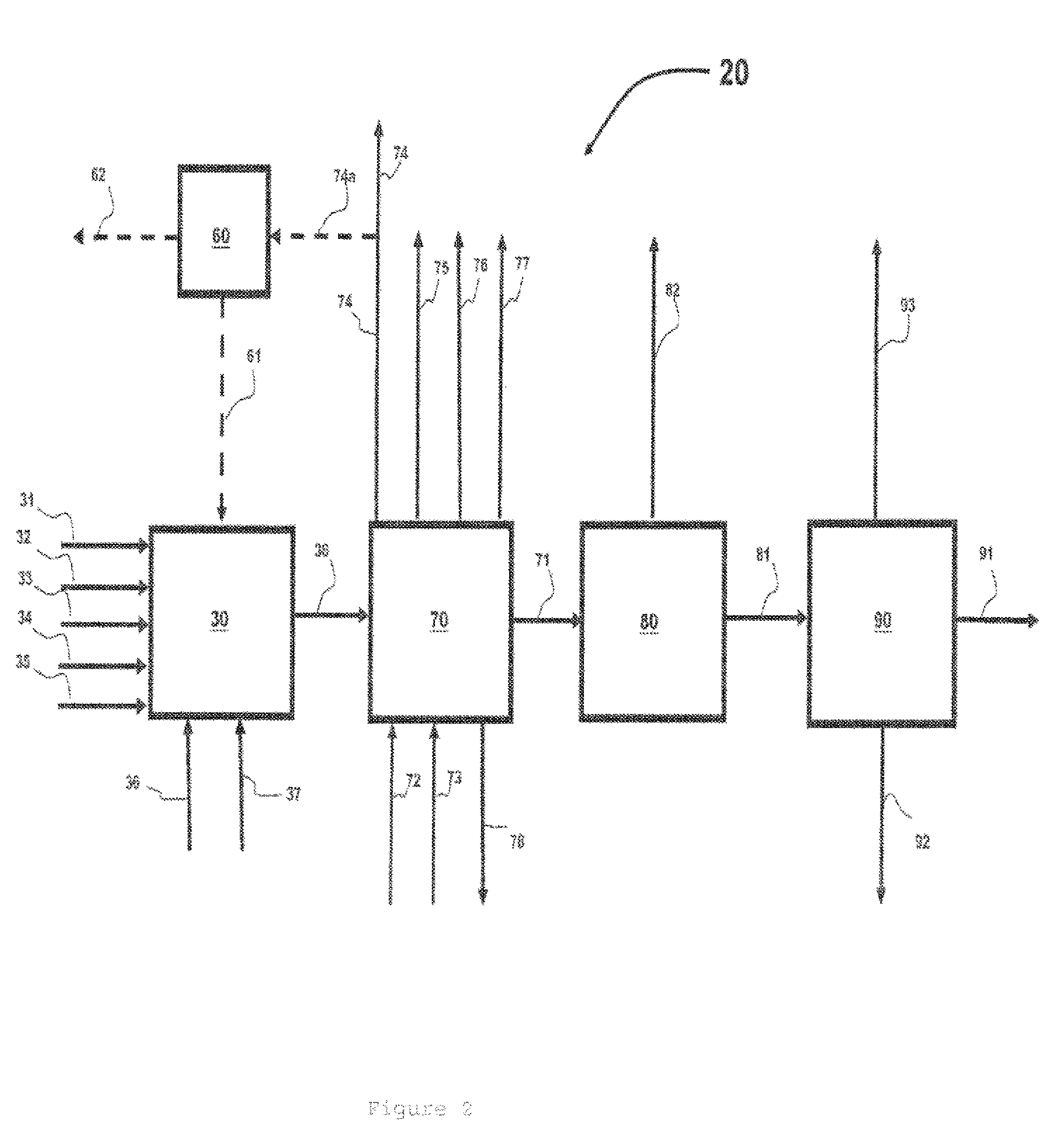
Resist composition and patterning process
InactiveUS20120288796A1High sensitivityHigh precisionPhotosensitive materialsPhotomechanical exposure apparatusChemistryResist
A resist composition is provided comprising a polymer comprising recurring units having a hydroxyl group substituted with an acid labile group, an onium salt PAG capable of generating a sulfonic acid, imide acid or methide acid, and an onium salt PAG capable of generating a carboxylic acid. A resist film of the composition is improved in dissolution contrast during organic solvent development, and from which a hole pattern having minimized nano-edge roughness can be formed via positive / negative reversal.
Owner:SHIN ETSU CHEM IND CO LTD
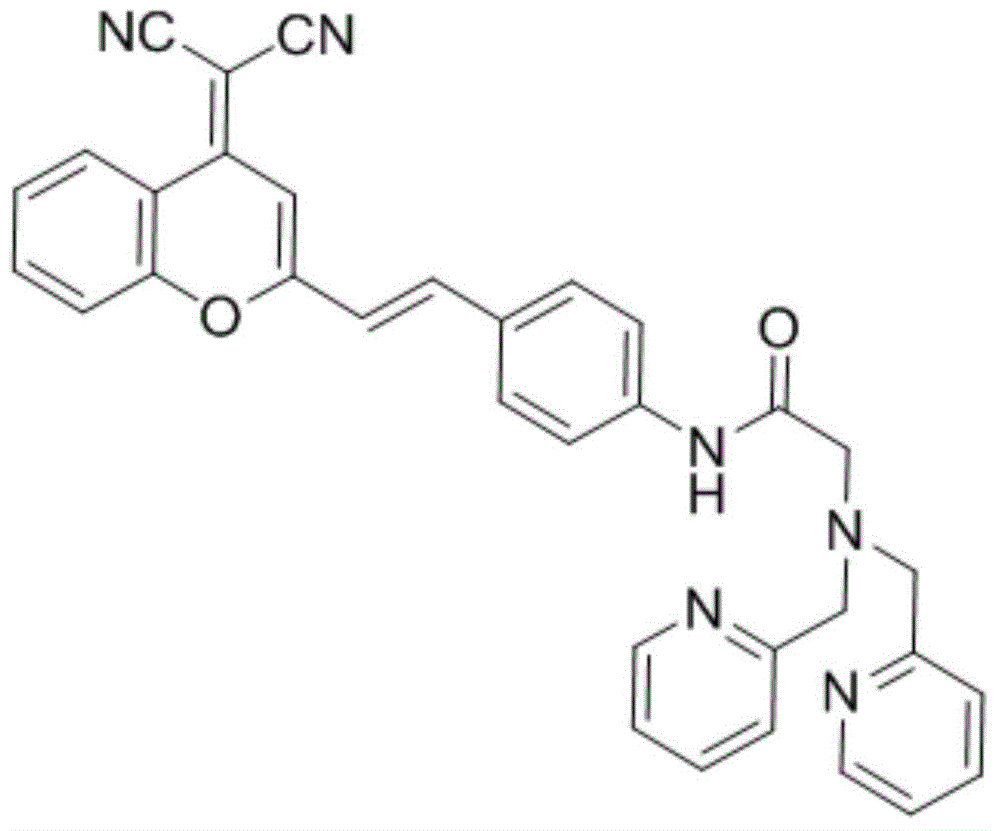
Ratio-type variant receptor mercury ion fluorescent probe and its preparation method and use
InactiveCN104804724AQuick responseImprove responsivenessOrganic chemistryFluorescence/phosphorescenceCadmium CationChemical stability
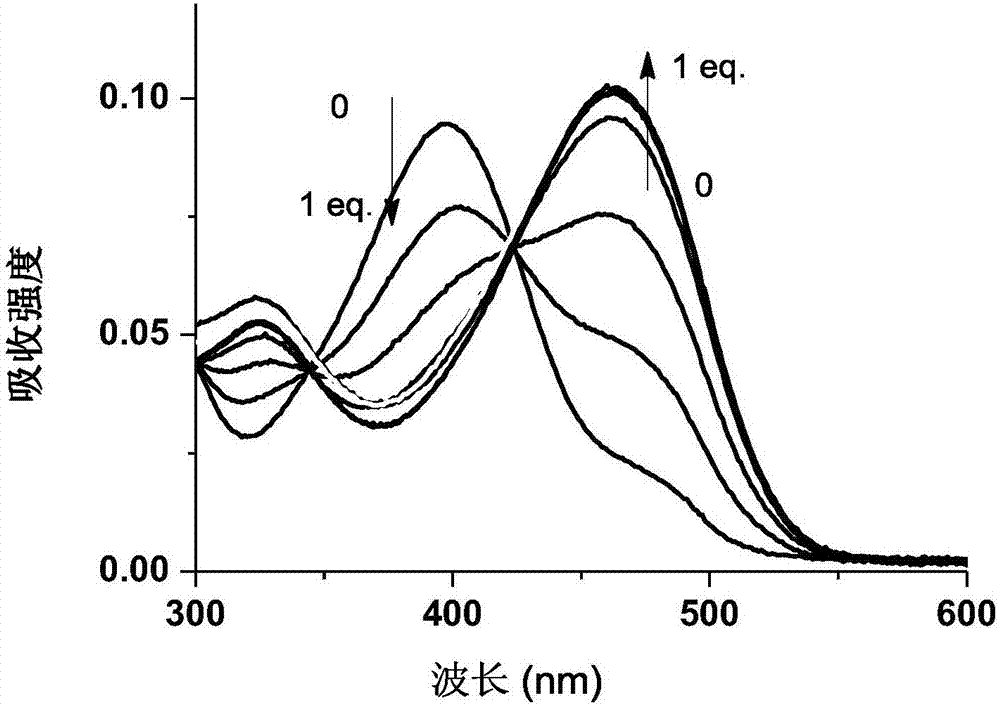
The invention discloses a ratio-type variant receptor mercury ion fluorescent probe and its preparation method and use. The ratio-type variant receptor mercury ion fluorescent probe has the advantages of simple synthesis processes, good light stability, the largest emission wavelength of 550nm and good chemical stability under neutral conditions. Through amide functional group tautomerism, the receptor has a deformation capability of combination of an imidic acid isomer and mercury ions and combination of an amide isomer structure and other metal ions so that the receptor has mercury ion specific combination selectivity. The probe with the mercury ion produces fluorescence spectrum red shift to 633nm and the fluorescence spectrum is enhanced by 9 times. After bonding to other metal ions especially such as congener zinc ion and cadmium ions, the probe has no obvious fluorescence change and has mercury ion specific fluorescence signal selectivity. The probe realizes mercury ion specific ratio identification, can be used in detection of mercury ions in cells and has a wide application prospect in detection of mercury ions in other organisms and environments.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for Producing Imidic Acid Salt
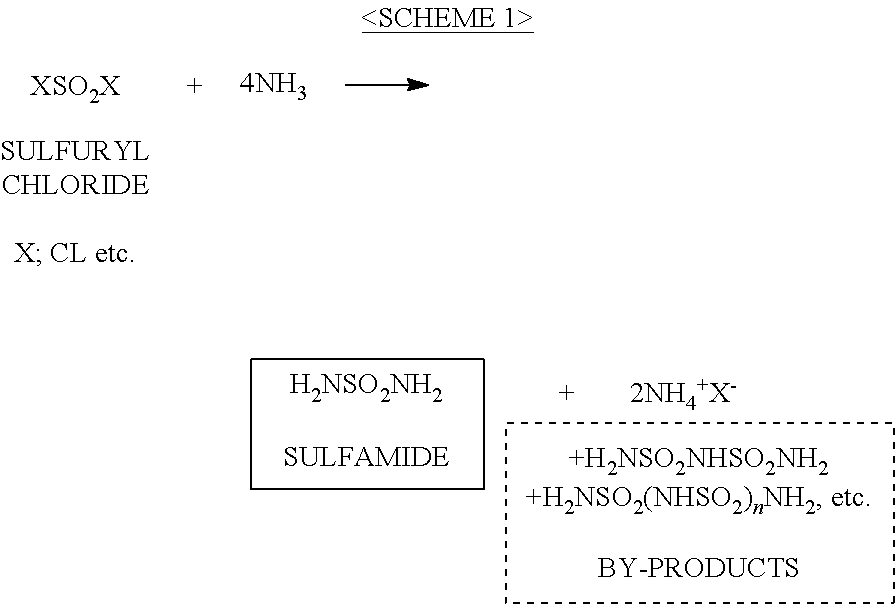
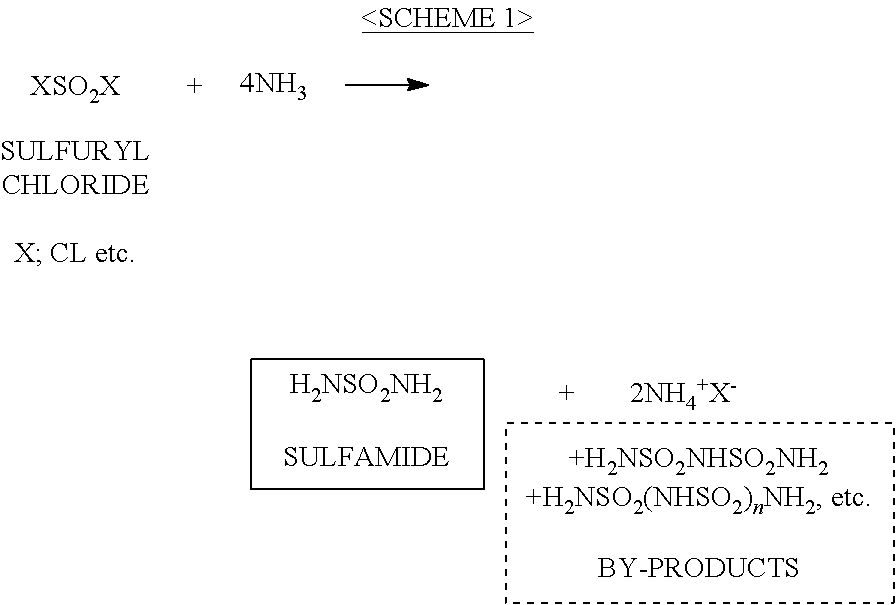
ActiveUS20120070358A1Easy to handleInhibition formationNitrosyl chloridePhosphorus halides/oxyhalidesAlkali metalHigh selectivity
To provide an imide salt represented by the formulawherein, R represents a halosulfonyl group (—SO2X1 where X1 is a halogen such as fluorine, chlorine, bromine and iodine) or dihalophosphoryl group (—POX2X3 where X2 and X3 are the same or different halogens such as fluorine, chlorine, bromine and iodine), and M represents an alkali metal;with high selectivity and high efficiency by using a low-cost starting material.In the production of an imide salt, an alkali metal fluoride, a sulfuryl halide or phosphoryl halide, and ammonia or an ammonium salt are reacted. According to this method, a desired imide salt can be produced with high yield, while greatly suppressing the production of a by-product.
Owner:CENT GLASS CO LTD
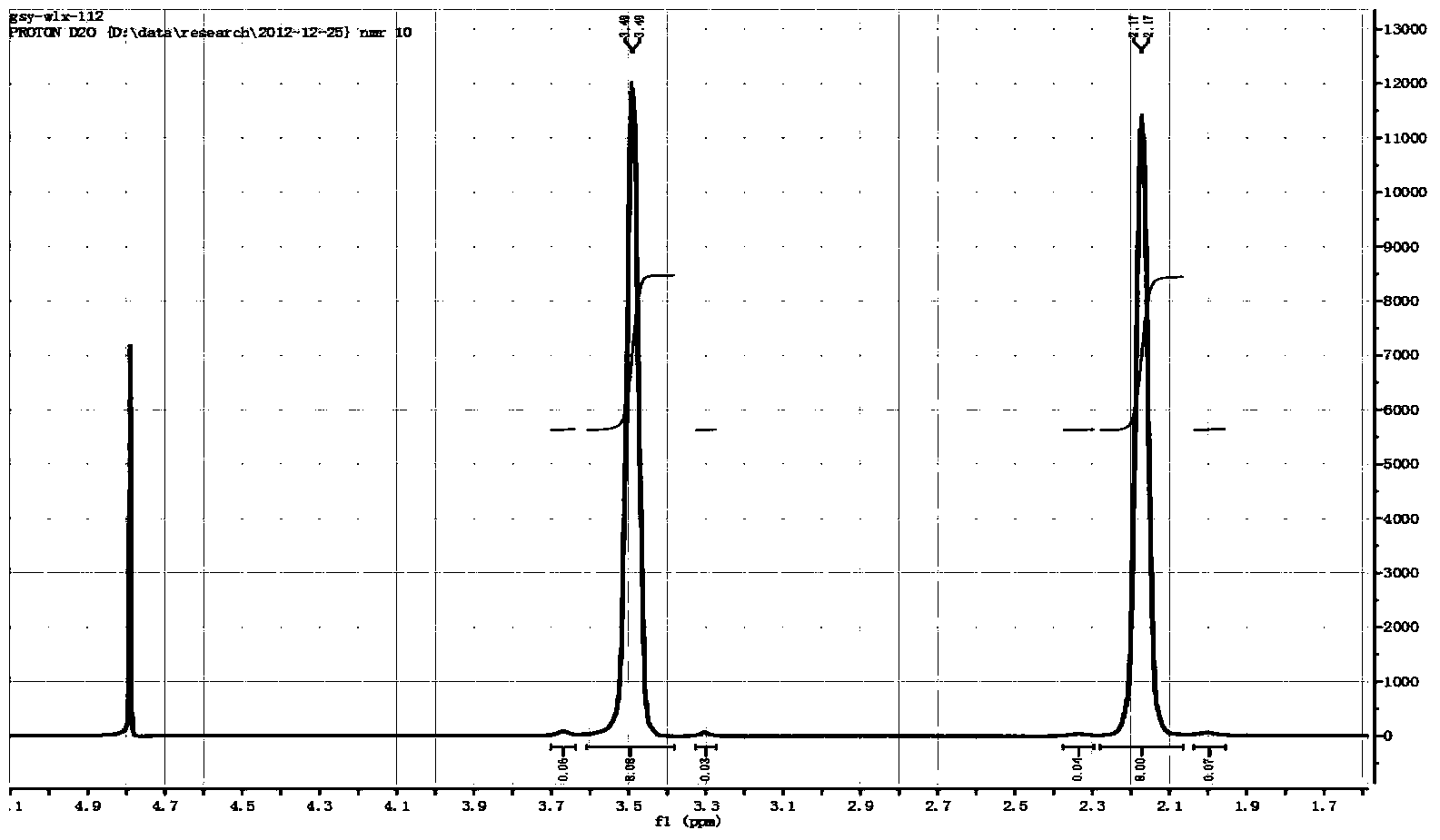
Preparation method of spiro-quaternary ammonium salt electrolyte
ActiveCN104387397ALow priceGood choiceHybrid capacitor electrolytesOrganic chemistryMorpholineStrong acids
The invention relates to a preparation method of a spiro-quaternary ammonium salt electrolyte. The preparation method is characterized by subjecting a cyclic amine, diol and a strong acid to condensation reaction at 120-180 DEG C for 8-16 hours in a polar organic solvent in the presence of a catalyst, and then purifying the reactant, thus obtaining the spiro-quaternary ammonium salt electrolyte, wherein the cyclic amine is any one of pyrrolidone, piperidine and morpholine; diol is 1,4-butanediol or 1,5-pentanediol; the strong acid is any one of tetrafluoroboric acid, trifluoromethanesulfonic acid and bis(trifluoromethanesulfonyl)imidic acid; the feed mole ratio of the cyclic amine to diol to the strong acid is 1 to (0.7-0.9) to (0.8-1). The preparation method has the advantages that the raw materials are cheap; the whole synthetic reaction is good in selectivity; the product has high yield and high purity; no halide ions are introduced in the reaction process, and the content of halogen in the obtained target product is less than 1ppm, so that the preparation method can meet the requirements of electrochemical energy storage electrolytes and is suitable for industrial production.
Owner:JIANGSU GUOTAI SUPER POWER NEW MATERIALS
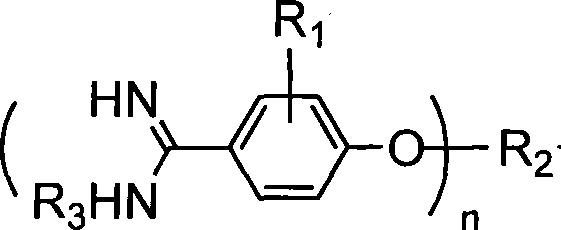
Method for synthesizing alkoxy aromatic amidine compounds
The invention discloses a method for synthesizing alkoxy aryl amidine compound, comprising the following steps: taking cyano-group phenol and alkylogen as raw materials, obtaining alkylphenyl ether by Williamson condensation, directly introducing chlorine hydride gas into the same reaction kettle to generate imidic acid ester hydrochloride, introducing inert gases to drive the chlorine hydride gas out, adding alkaline air or organic amine to prepare amidine hydrochloride by amination and adding alkali to remove chlorine hydride to obtain the target compound. In the method, identical solvent is used as medium and steps of condensation, addition, amination and the like are combined to one step in a single reaction kettle, so that complex intermediate treatment process is excluded and process is simplified; and meanwhile, loss of the solvent can be reduced and yield of product is improved.
Owner:南京枫华投资管理有限公司
Actinic-ray- or radiation-sensitive resin composition, actinic-ray- or radiation-sensitive film and method of forming pattern
ActiveUS20150338736A1Good influenceReduce fluorine contentSteroidsPhotomechanical exposure apparatusPolymer scienceMethyl group
Provided is an actinic-ray- or radiation-sensitive resin composition including a resin (A) and any of compounds (B) of general formula (I) below. (In general formula (I), Rf represents a fluorine atom or a monovalent organic group containing at least one fluorine atom; R1 represents a hydrogen atom or a monovalent substituent containing no fluorine atom; X1 represents a monovalent organic group having at least two carbon atoms, or a methyl group in which a substituent other than a fluorine atom is optionally introduced, provided that X1 may be bonded to R1 to thereby form a ring; and Z represents a moiety that when exposed to actinic rays or radiation, is converted to a sulfonic acid group, an imidic acid group or a methide acid group.)
Owner:FUJIFILM CORP
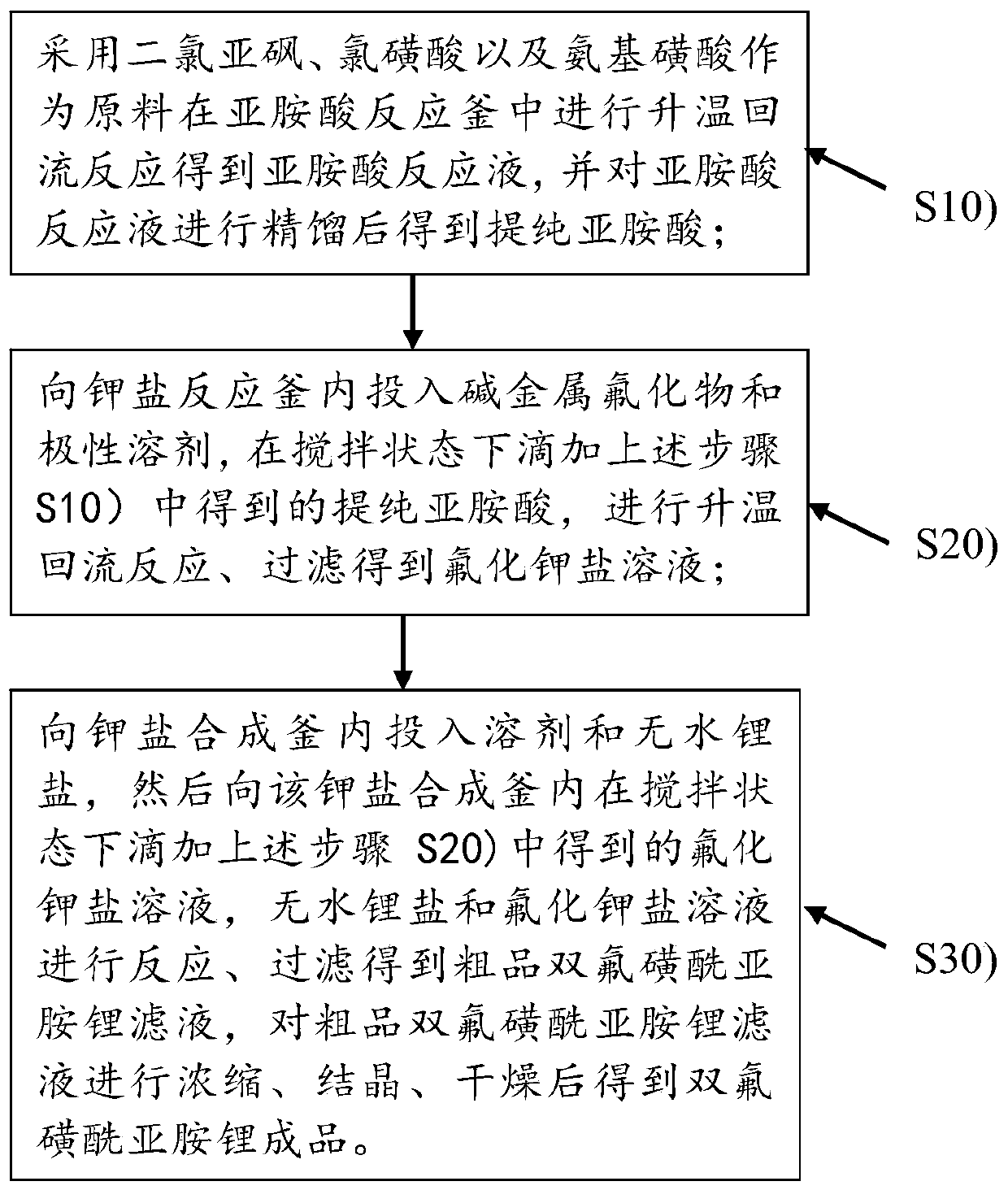
Preparation method of lithium bis (fluorosulfonyl) imide and lithium bis (fluorosulfonyl) imide
The invention discloses a preparation method of lithium bis (fluorosulfonyl) imide and the lithium bis (fluorosulfonyl) imide. Thionyl chloride, chlorosulfonic acid and sulfamic acid which are low inraw material cost and easy to obtain are used as raw materials; imidic acid is prepared under the condition of heating reflux reaction, potassium fluoride which is also used as a raw material and is low in cost and easy to obtain is used as a raw material to react with the imidic acid to obtain sylvite, potassium fluoride salt and anhydrous lithium salt react in a solvent to generate the lithium bis (fluorosulfonyl) imide, and the preparation process is low in energy consumption and has operation safety. And meanwhile, purification process treatment is carried out in each step, so that the high-purity and high-yield lithium bis (fluorosulfonyl) imide can be finally obtained, and the method is very suitable for being used as a process route for batch production of the lithium bis (fluorosulfonyl) imide.
Owner:CHANGSHU XINHUA CHEM
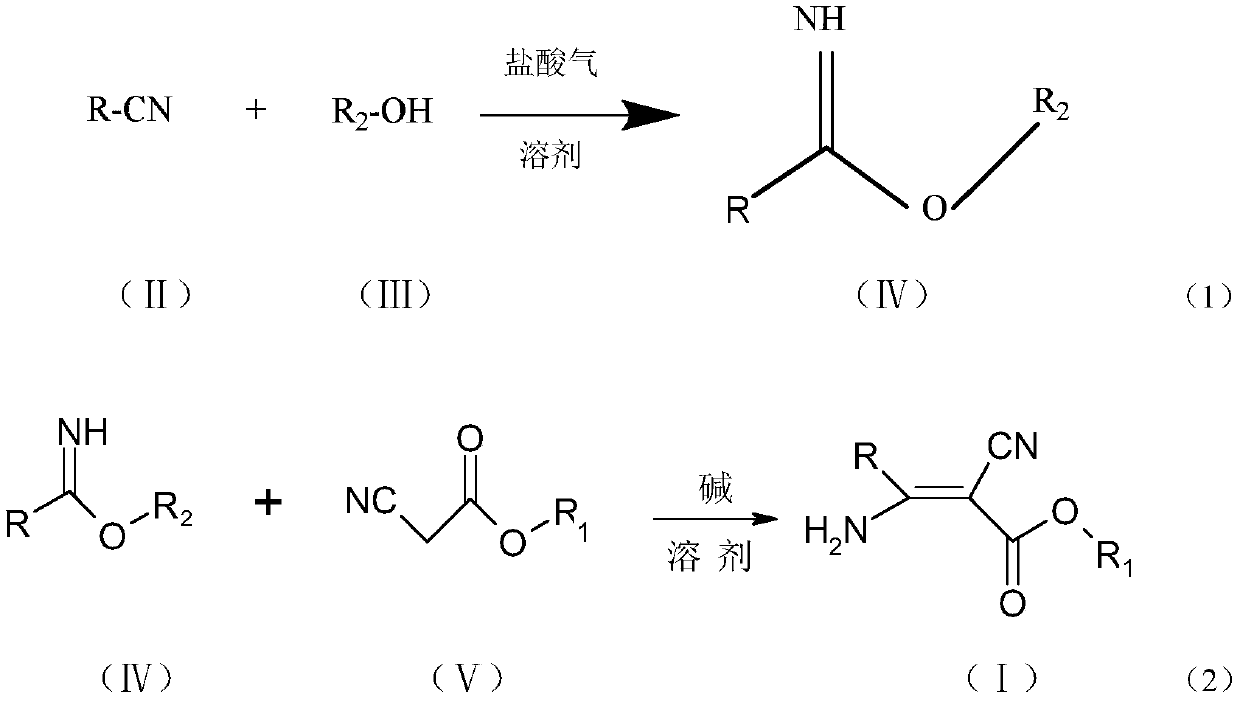
3-pyridyl-3-amino-2-cyanoacrylate compound, and preparation method and application thereof
The invention discloses a 3-pyridyl-3-amino-2-cyanoacrylate compound, and a preparation method and application thereof. The structure of the compound is shown in a general formula (1). The preparationmethod of the compound comprises the following steps: taking a compound shown in a formula (II) as a raw material to carry out a reaction with a fatty alcohol in an organic solvent in the presence ofhydrogen chloride to obtain pyridyl imidate, and carrying out a reaction on the pyridyl imidate with cyanoacetate in an organic solvent under catalysis of a catalyst for condensation to prepare the 3-pyridyl-3-amino-2-cyanoacrylate compound. The compound has bactericidal activity and can be used for preventing and treating diseases.
Owner:JIANGSU PESTICIDE RES INST
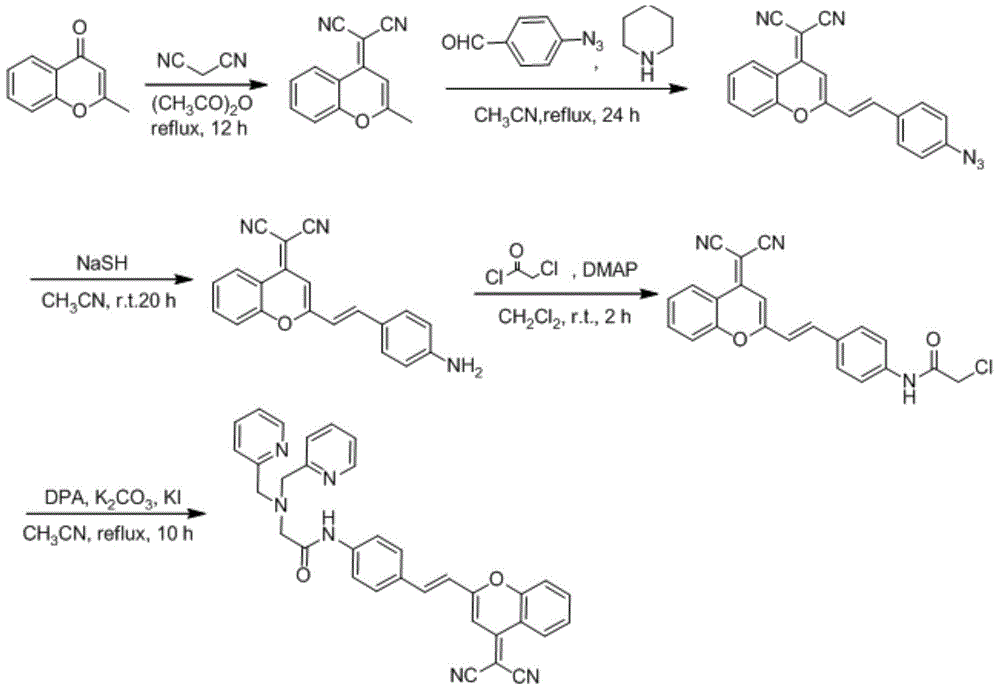
Cadmium ion fluorescence probe, preparation method and applications thereof
InactiveCN107501252AQuick responseImprove solubilityOrganic chemistryFluorescence/phosphorescenceFluorophoreMetal
The present invention provides a cadmium ion fluorescence probe, a preparation method and applications thereof. According to the present invention, the probe has specific binding selectivity and fluorescence response to cadmium ions; 4-amno-7-nirobenzoxadiazole is used as a fluorophore and amide DPA is used as a receptor in the probe, and the acceptor can have the variation ability through the tautomerism of the amide functional group, wherein the isomer of the imidic acid binds the cadmium ions, and the structure of the amide isomer binds other metal ions so as to provide the specific binding selectivity to the cadmium ions; and the unique identification on the cadmium ions by the probe is achieved, the obtained probe can be used for detecting the cadmium ions in cells, and can further be used for the cadmium ion detection in other organisms and environments due to the wide pH value working range.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Wavelength-selective absorption filter
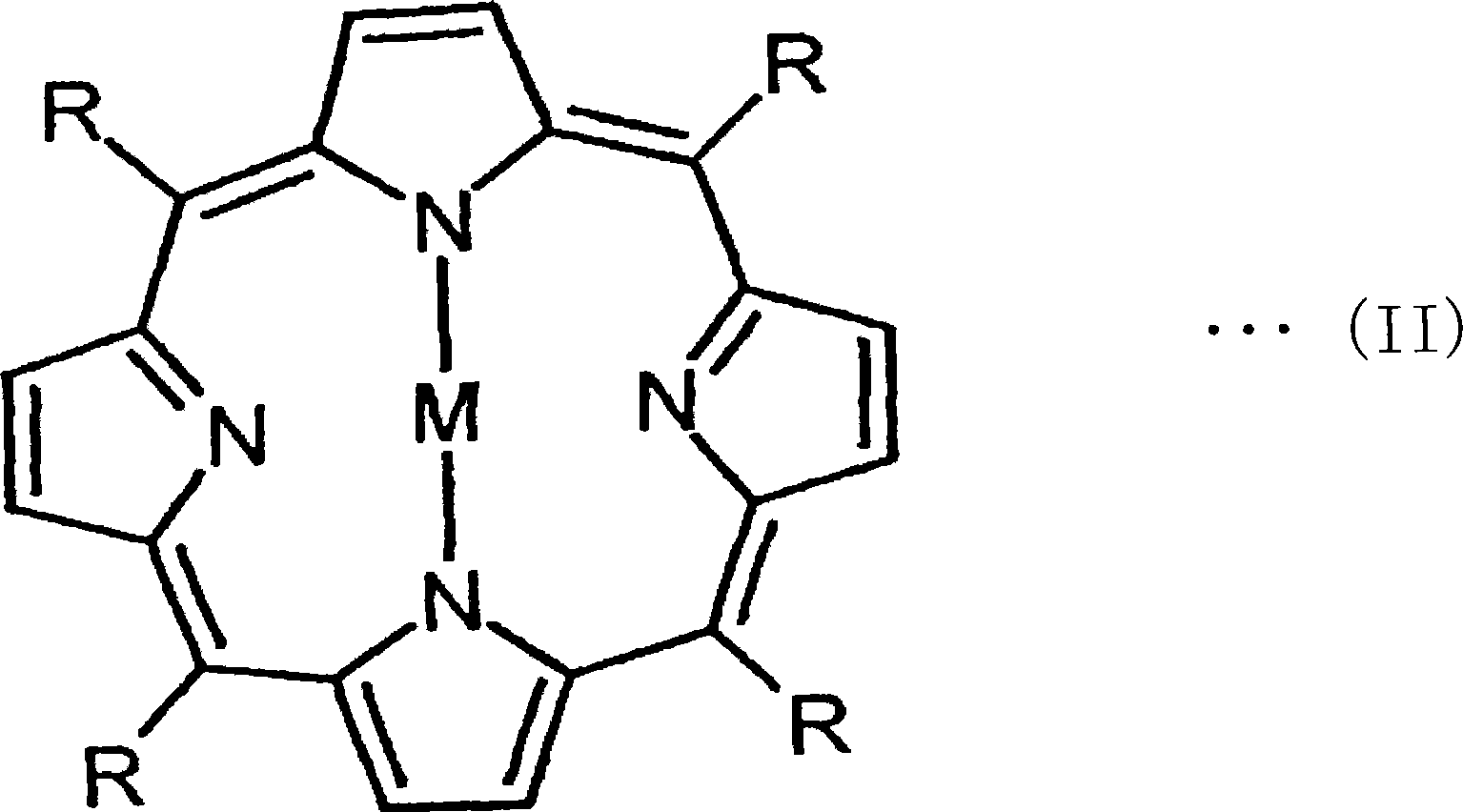
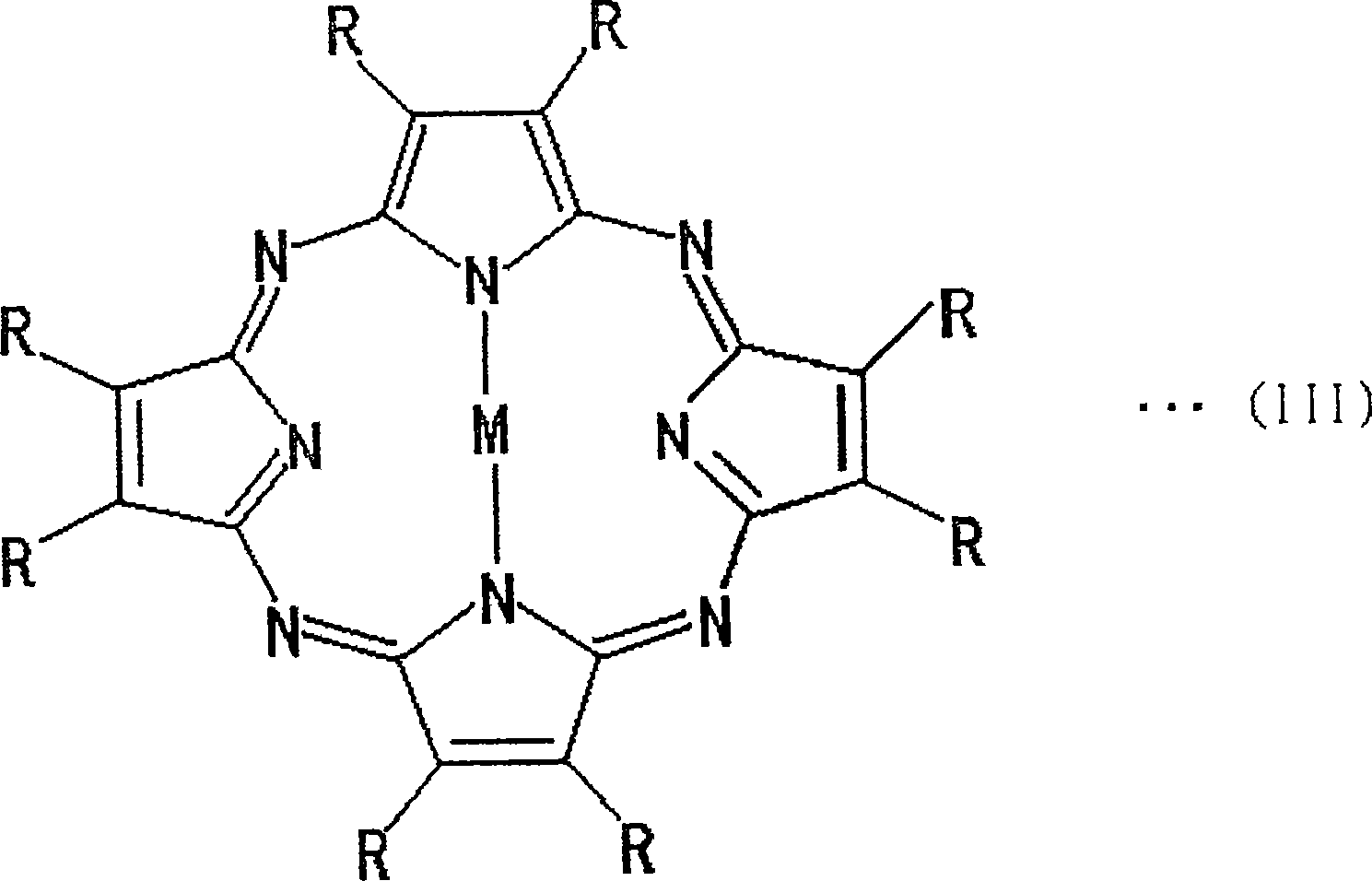
InactiveCN101076746ALittle change over timeAvoid misuseOther chemical processesSynthetic resin layered productsAbsorption filterPorphyrin
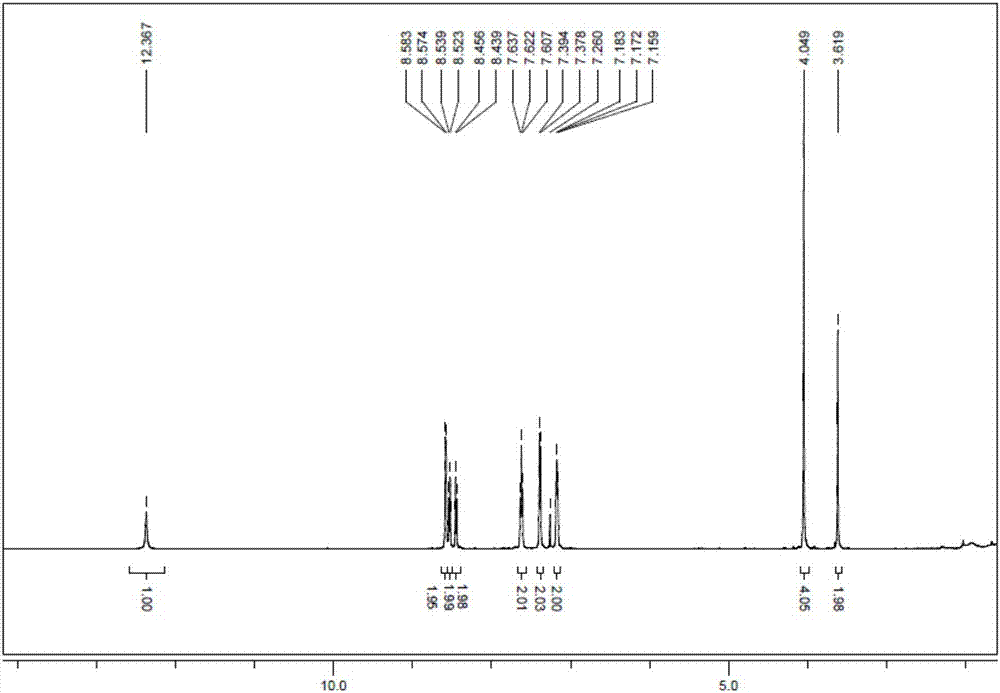
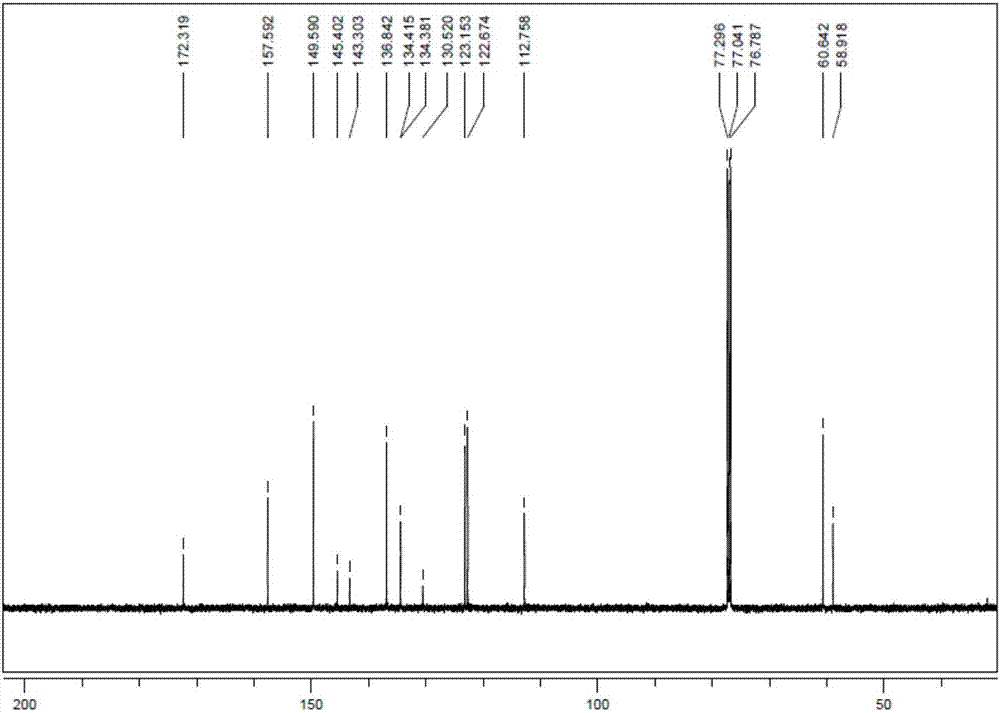
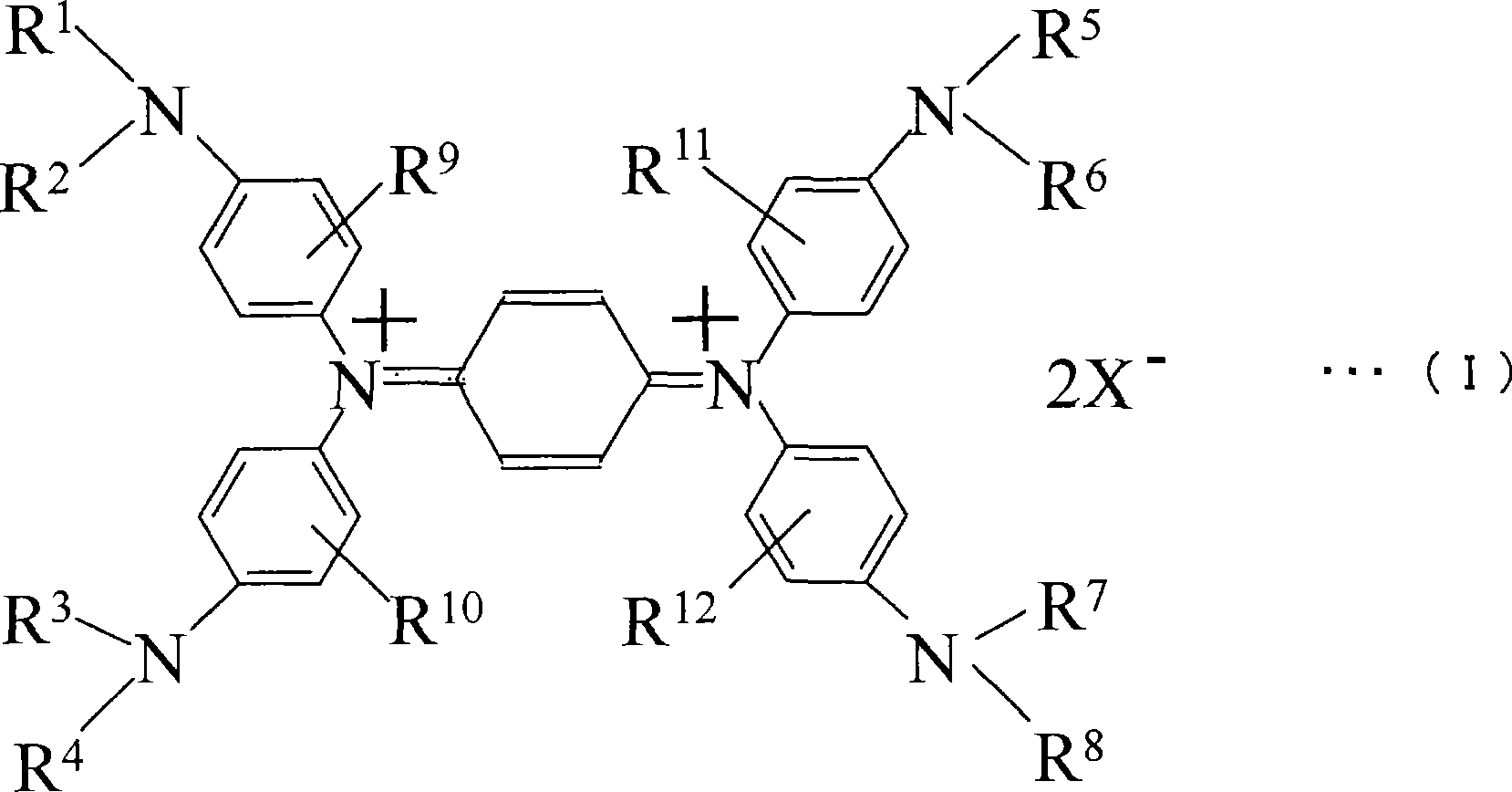
This invention provides a wavelength-selective absorption optical filter that exhibits high level and broad absorption in a near infrared region, further absorbs neon light, and exhibits high light transmittance in other visible light region. The wavelength-selective absorption optical filter is less likely to cause a change in optical properties with the elapse of time and is highly durable. The wavelength-selective absorption optical filter comprises a transparent base material and a wavelength-selective absorption layer having a single or multilayer structure stacked on the base material. The wavelength-selective absorption layer comprises a resin, an near infrared absorptive coloring matter (A) and a coloring matter (B), and the wavelength-selective absorption optical filter has maximum absorption at a wavelength of 800 to 1200 nm and a wavelength of 550 to 620 nm. The wavelength-selective absorption optical filter is characterized in that one type of the near infrared absorptive coloring matter (A) is an aromatic diimmonium coloring matter (a) of which the counter ion is bis(trifluoromethansulfonyl)imidic acid, and one type of the coloring matter (B) is a porphyrin coloring matter or azaporphyrin coloring matter (b).
Owner:TOYO TOYOBO CO LTD
Method for improving beta-glucosidic bond stereoselectivity through bis(trifluoromethane sulfonimide) reagent activation glycosylation reaction
InactiveCN105541933AReduce dosageMild reaction conditionsSugar derivativesSugar derivatives preparationLeaving groupBiological activation
The invention discloses a method for improving beta-glucosidic bond stereoselectivity through a bis(trifluoromethane sulfonimide) reagent activation glycosylation reaction. According to the method, under the action of a catalytic amount of bis(trifluoromethane sulfonimide) reagent, a glycosyl donor with a leaving group being the trichloroacetic imidic acid ester group or acetylenic acid ester group is subjected to coupling with different glycosyl receptors, the using amount of the bis(trifluoromethane sulfonimide) reagent is small, the reaction condition is mild, the product yield is high, and a beta-glycosylation product can be obtained in a high-stereoselectivity mode.
Owner:SHAANXI NORMAL UNIV
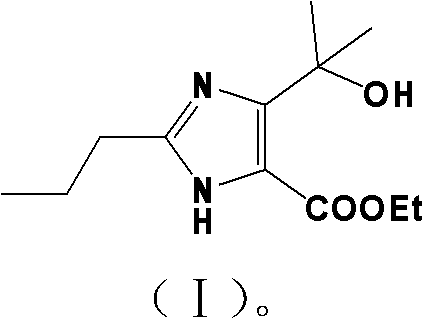
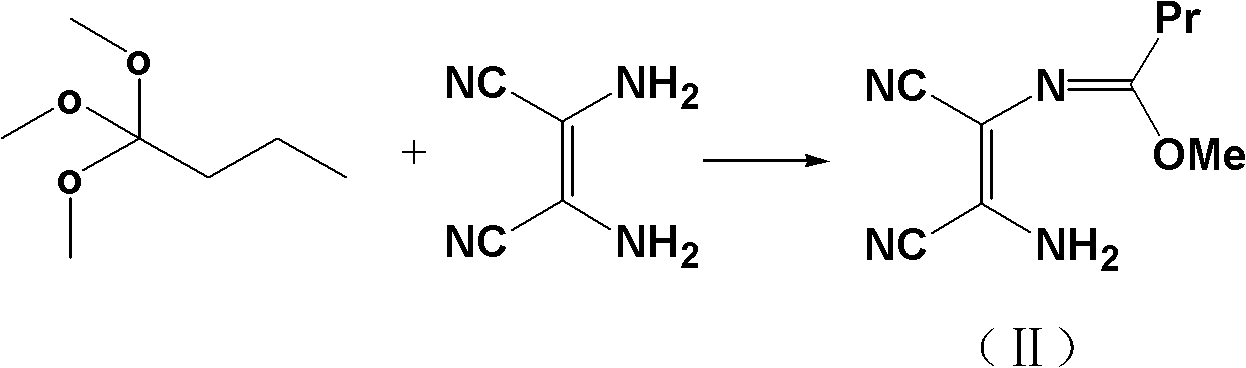
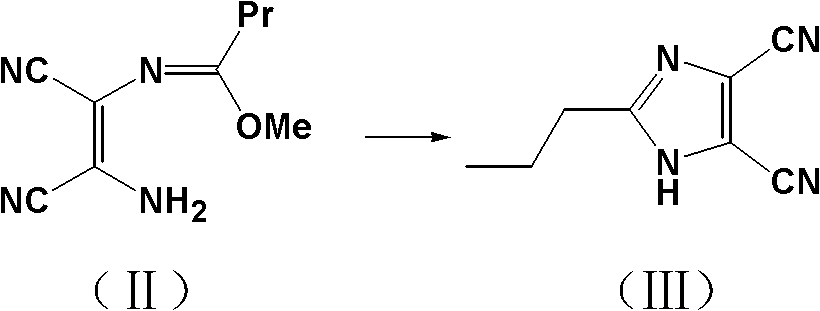
Method for synthesizing 4-(1-hydroxyl-1-methylethyl)-2-propyl iminazole-5-carboxylic ethyl ester
ActiveCN102060778AAvoid wastingGood decolorization effectOrganic chemistryGrignard reagentCarboxylic acid
The invention discloses a method for synthesizing 4-(1-hydroxyl-1-methylethyl)-2-propyl iminazole-5-carboxylic ethyl ester, which comprises the steps of: using trimethyl orthobutyrate and diaminomaleonitrile as raw materials, and performing backflow reaction to prepare an imidic acid ester intermediate in presence of alcohols solvents; adding active carbon, continuously performing backflow for 1 hour, and filtering while the mixture is hot; cooling the filtrate to 0-15 DEG C, and introducing ammonia to prepare 2-propyl iminazole-4,5-dinitrile; adding the 2-propyl iminazole-4,5-dinitrile to a mixed solution of hydrochloric acid and ethanol and esterfying in backflow state to prepare 2-propyl iminazole-4,5-diethyl dicarboxylate; and performing reaction of 2-propyl iminazole-4,5-diethyl dicarboxylate and a Grignard reagent to prepare the 4-(1-hydroxyl-1-methylethyl)-2-propyl iminazole-5-carboxylic ethyl ester. By adopting the synthesizing method, the total yield of the synthesized 4-(1-hydroxyl-1-methylethyl)-2-propyl iminazole-5-carboxylic ethyl ester is 81% which is obviously superior to the total yield (55%) of the traditional references; and the invention has the advantages of concise process and low cost and is suitable for industrial production.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Process for preparing divinylarene dioxides
A process for preparing a divinylarene dioxide including reacting (a) at least one divinylarene; (b) at least one peroxycarboximidic acid; (c) at least one solvent; and (d) at least one basic compound, under conditions to form a reaction mixture containing a divinylarene dioxide product; and then separating the divinylarene dioxide product from the other reaction mixture components to obtain a purified divinylarene dioxide product.
Owner:BLUE CUBE IP
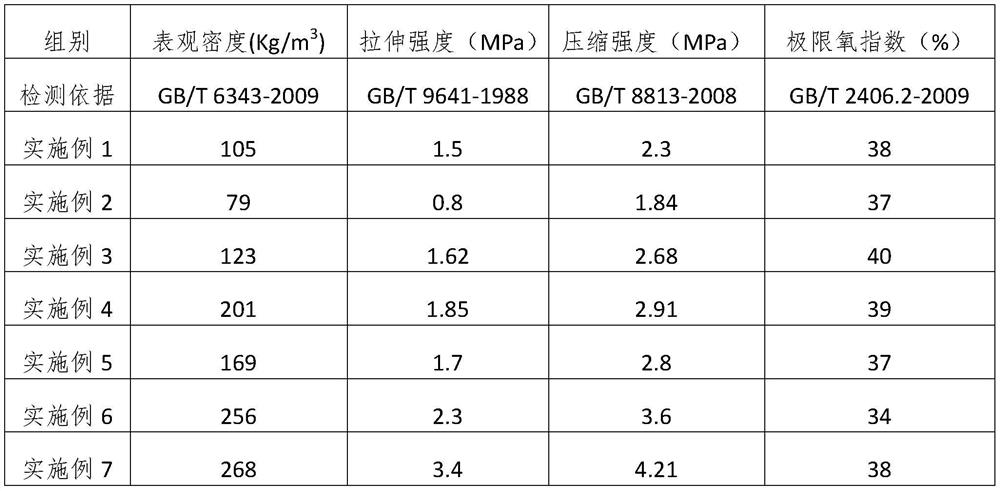
Production method of hard polyimide foam material
ActiveCN111793237AImprove mechanical propertiesImprove flame retardant performanceImidePolymer science
The invention belongs to the technical field of plastic foaming, and particularly relates to a production method of a hard polyimide foam material. The production method comprises the following steps:mixing imidic acid precursor powder and imide ester precursor powder, adding a foam stabilizer and / or a foaming agent, carrying out uniform mixing, and carrying out foaming die pressing to obtain thehard polyimide foam material, wherein the imidic acid precursor powder is prepared by a reaction of dianhydride and diamine, and the imide ester precursor powder is prepared by reacting dianhydride with fatty alcohol to obtain diacid diester and reacting the diacid diester with isocyanate. The material has good mechanical properties, flame retardancy, high and low temperature resistance and the like, and can be used as a structural member in the fields of aerospace, marine ships and warships and the like.
Owner:GUIZHOU AEROSPACE TIANMA ELECTRICAL TECH
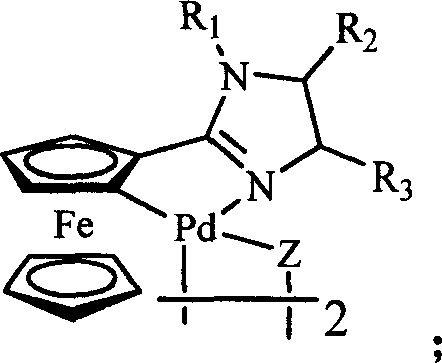
Ferrocenyl imidazoliny palladium compound, its preparation method and its uses in catalytic synthesis of coupling product
InactiveCN101007825AGood structural controlHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrocarbon condensationHalidePalladium compound
The invention discloses a ferrocenyl imidazoline palladacycle complexe, the preparation method and its application in catalizing coupling product. The general formula of compound is in the right. The method comprises: carrying out reaction with ferrocenyl imidic acid methyl ester hydrochlorate and vicinal diamine, getting ferrocenyl imidazoline compound through post treatment; carrying out reaction with mentioned compound above with palladium lithium halides and NaOAc in organic solvent while being stirred at room temperature, and getting final product. The catalytic synthesis of coupling product with siad compound comprises following steps: dissloving catalyst, aryl boracic acid, alkali and halogenated aromatic compound in organic solvent, heating for reaction, cooling for extraction, purifying and getting coupling product. The invention is characterized by high catalytic property, environmental- friendly, insenstive to aor and water, good thermal stability of non-phosphine ligand, easy processing route, temperate reaction condition, and high specificity.
Owner:ZHENGZHOU UNIV
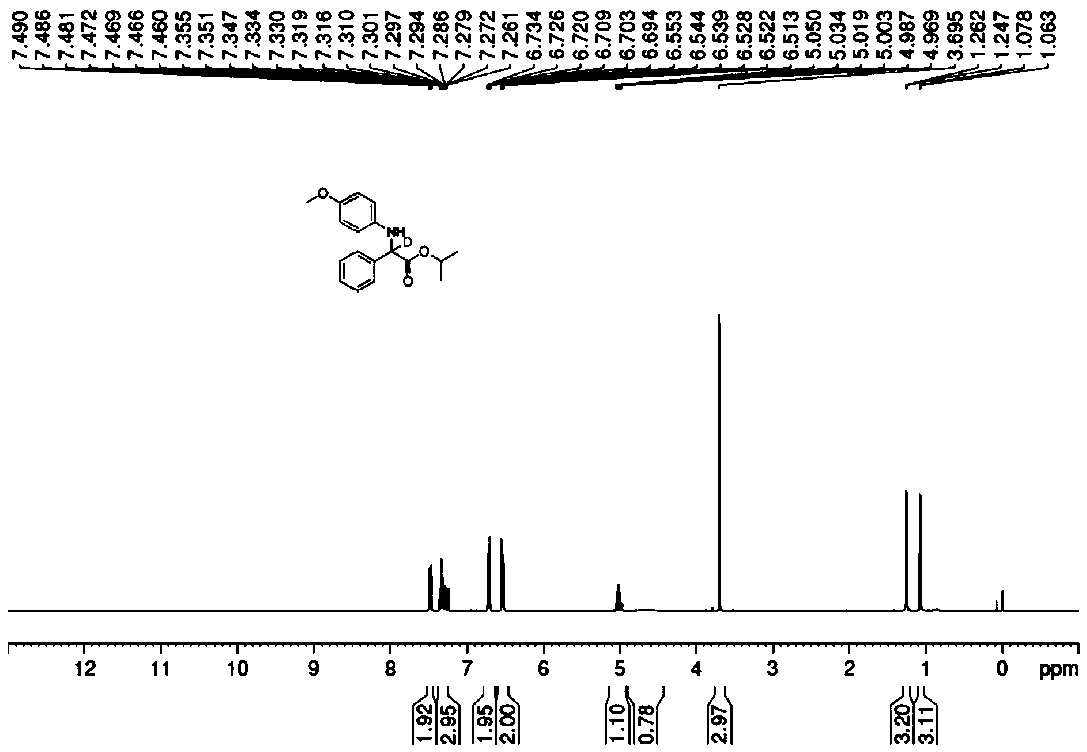
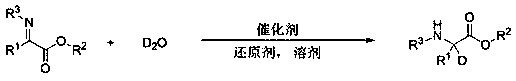
Method for preparing chiral amino acid ester and chiral deuterated amino acid ester
InactiveCN110885294AEasy to operateGentle operationOrganic compound preparationAmino-carboxyl compound preparationImino acidPerylene derivatives
A method for efficiently preparing chiral amino acid ester and chiral deuterated amino acid ester is disclosed. Water or deuterated water is adopted as a hydrogen source or a deuterium source to achieve asymmetric transfer hydrogenation or deuteration of imino acid ester in an inert gas atmosphere to obtain the chiral amino acid ester and chiral deuterated amino acid ester derivatives. The methodfor preparing the chiral amino acid ester and chiral deuterated amino acid ester, which is simple and convenient to operate, mild, efficient and green, is provided.
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA
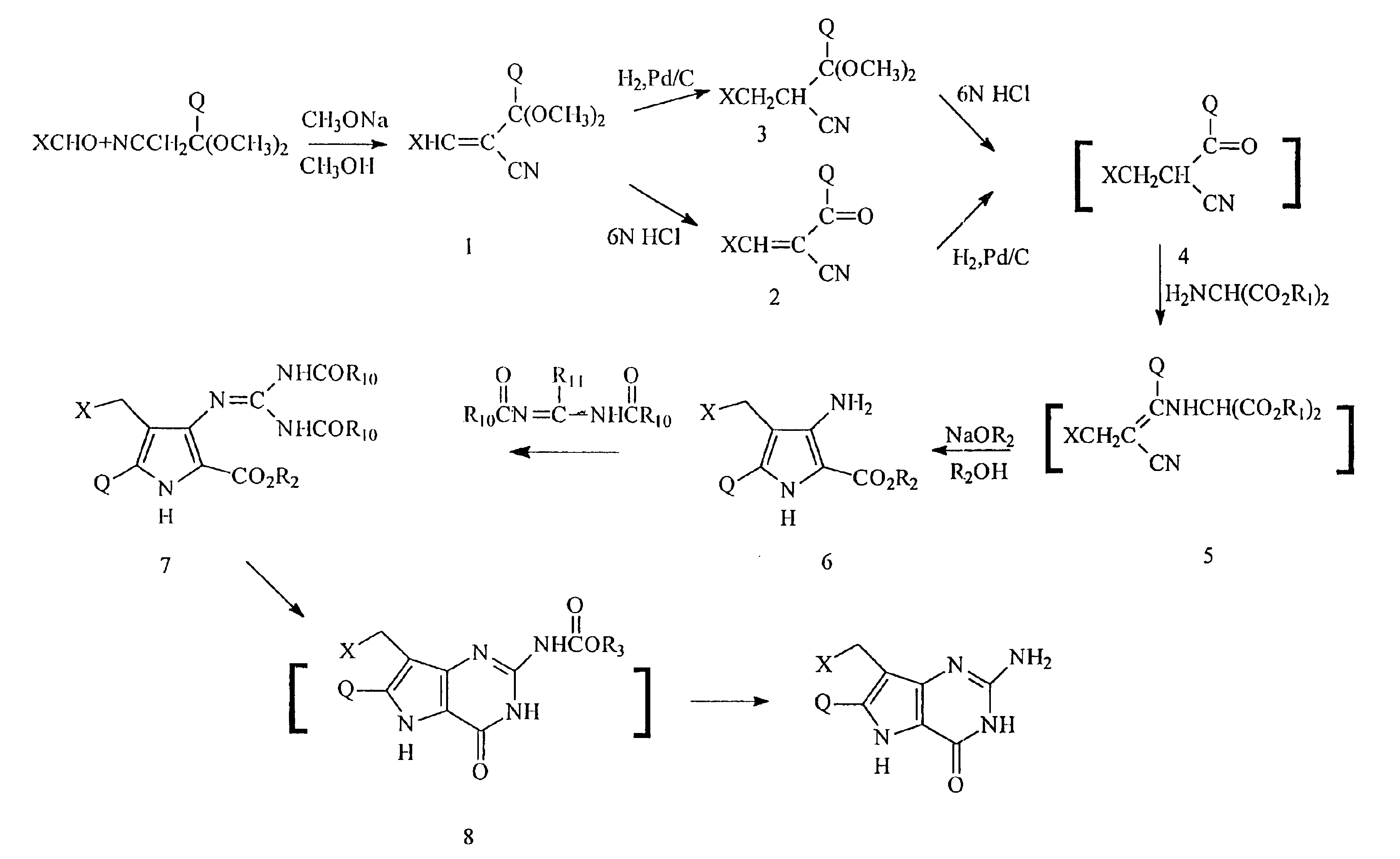
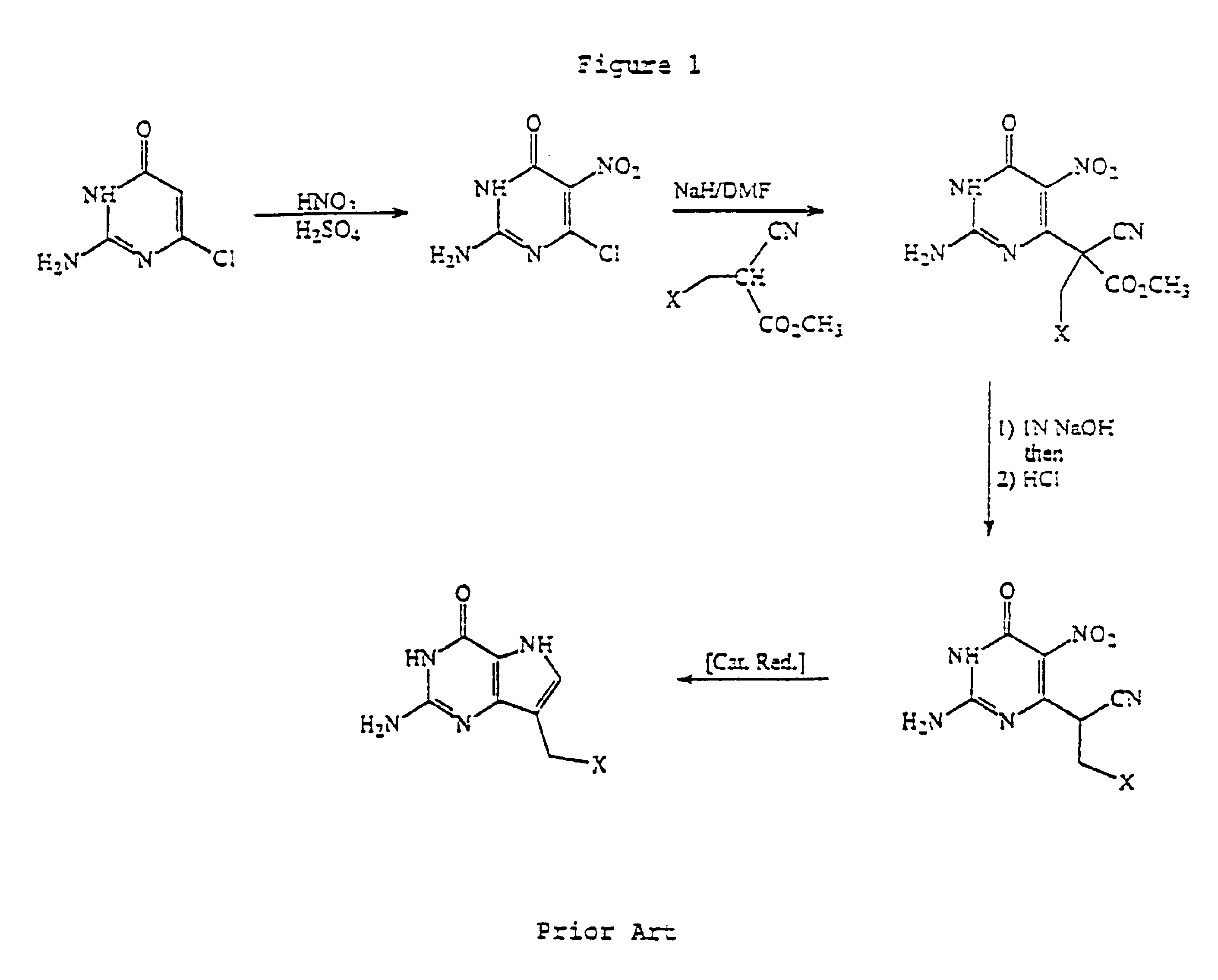
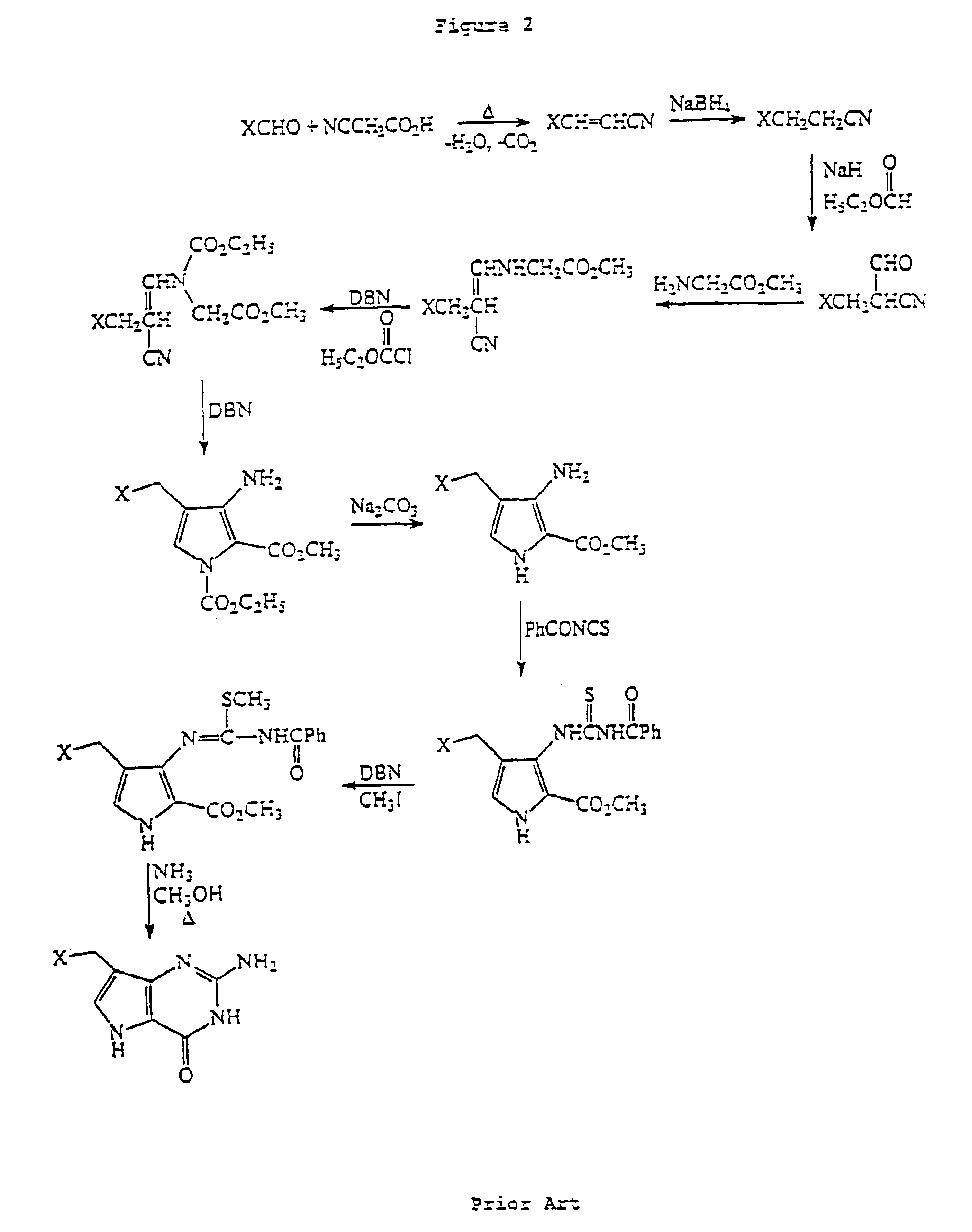
Process for the preparation of 9-deazaguanine derivatives
InactiveUS6946554B2Easy to operateHigh yieldBiocideOrganic chemistryAlkaline earth metalTrifluoroacetic acid
Derivatives of 9-deazaguanine of the formula I: are prepared by reacting an aldehyde or ketone of the formula II: with a dialkylaminomalonate to produce an enamine. The enamine is then reacted with a base to produce a pyrrole represented by the formula: The pyrrole is reacted with a compound represented by the formula R3OC(O)N═C(Z)NHC(O)OR3 or a derivative of carbamimidoic acid to provide a protected guanidine compound. The guanidine compound is converted to the desired deazaguanine by reaction with 1) trifluoracetic acid or 2) C1-C4 alkoxide or alkali metal or alkaline earth metal hydroxide followed by neutralization with an acid.
Owner:BIOCRYST PHARM INC
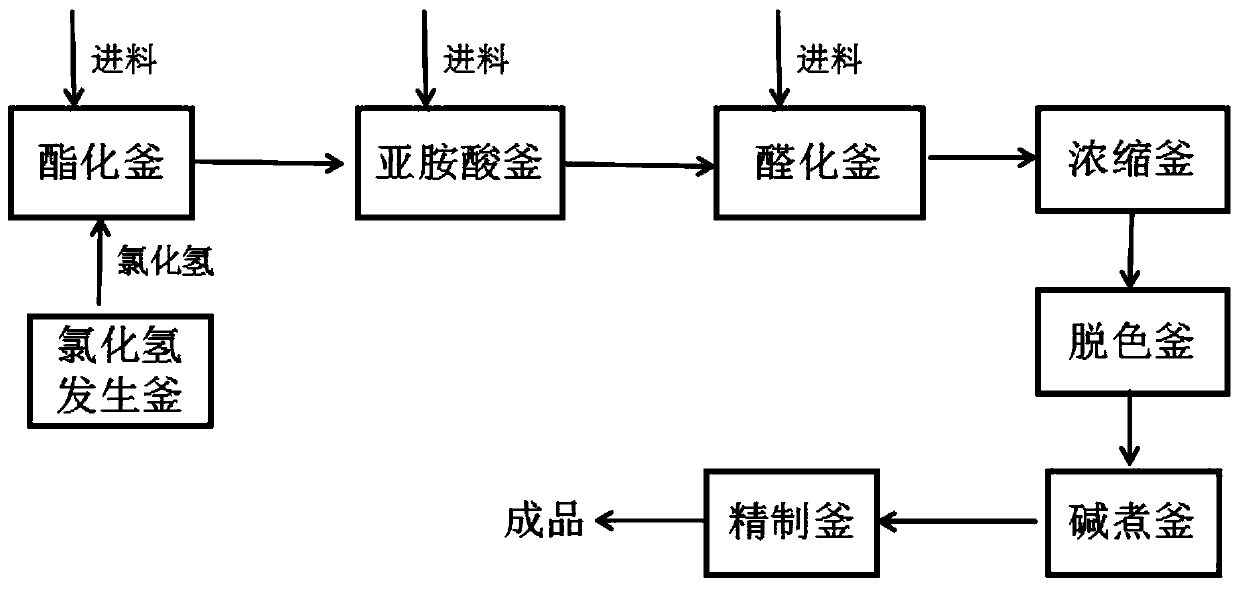
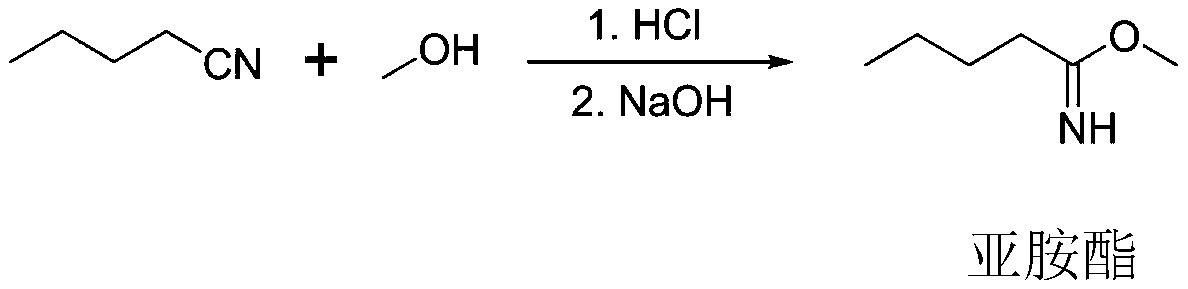
Production process of imidazole aldehyde
The invention provides a production process of imidazole aldehyde. Imiline is prepared by using valeronitrile and methanol as raw materials, glycine and methanol are added to prepare imidic acid, anda further reaction is conducted with phosphorus oxychloride and DMF to obtain the imidazole aldehyde. By controlling reaction conditions and the feed ratio of the raw materials, the formation of by-products is reduced, and the yield is improved. A decoking and decolorization reaction and an activated carbon decolorization reaction are adopted, the solubility change of the imidazole aldehyde underdifferent pH conditions is used for purifying a product, and finally, the imidazole aldehyde with a purity of more than 99.2% is obtained by recrystallization.
Owner:江西三元药业有限公司
Method for producing imidic acid salt
ActiveUS8840856B2Easy to handleInhibition formationNitrosyl chloridePhosphorus halides/oxyhalidesPhotochemistryAlkali metal
To provide an imide salt represented by the formulawherein, R represents a halosulfonyl group (—SO2X1 where X1 is a halogen such as fluorine, chlorine, bromine and iodine) or dihalophosphoryl group (—POX2X3 where X2 and X3 are the same or different halogens such as fluorine, chlorine, bromine and iodine), and M represents an alkali metal;with high selectivity and high efficiency by using a low-cost starting material.In the production of an imide salt, an alkali metal fluoride, a sulfuryl halide or phosphoryl halide, and ammonia or an ammonium salt are reacted. According to this method, a desired imide salt can be produced with high yield, while greatly suppressing the production of a by-product.
Owner:CENT GLASS CO LTD
Preparation method of bis(fluorosulfonyl)imide salt
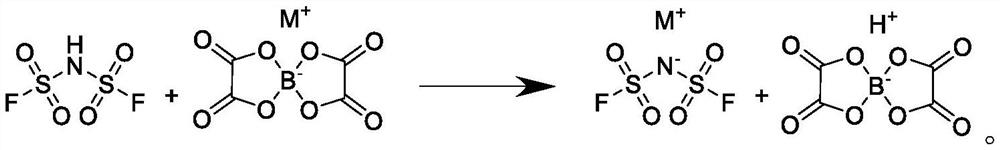
ActiveCN114031053AImprove solubilityPromote precipitationNitrosyl chlorideAmidosulfonic acidImideBorate salt
The invention discloses a preparation method of bis(fluorosulfonyl)imide salt. The preparation method comprises the following steps: firstly, mixing bis(fluorosulfonyl)imidic acid and an inert solvent to obtain a bis(fluorosulfonyl)imidic acid inert solvent solution; and dissolving bis(oxalato)borate with a benign solvent, dropwise adding the bis(oxalato)borate into the bis(fluorosulfonyl)imidic acid inert solvent solution for a reaction, and carrying out direct filtering after the reaction is finished to obtain the bis(fluorosulfonyl)imidic salt. According to the preparation method, byproducts are few, a prepared product and the byproducts are easy to separate, product yield is high, and product quality is excellent.
Owner:ZHANGJIAGANG HUASHENG CHEM CO LTD
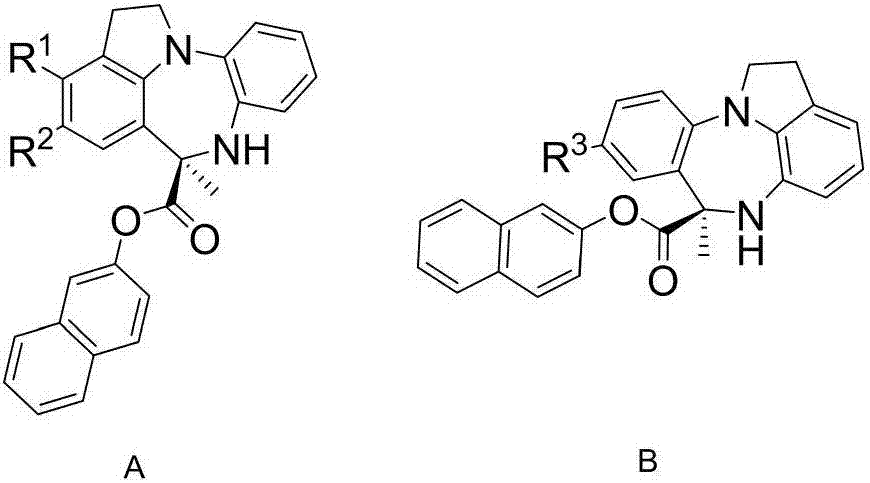
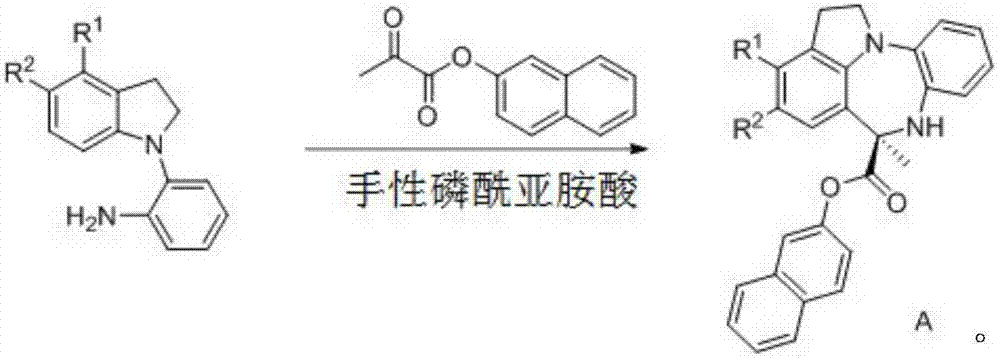
Chiral benzodiazepine compound and synthesis method thereof
The invention discloses a chiral benzodiazepine compound and a synthesis method thereof. The method comprises the step of reacting substituted arylamine and pyruvic acid-2-naphthyl ester under the catalysis of chiral phosphoryl imidic acid. The method disclosed by the method has the advantages that the reaction condition is mild, reaction under normal temperature is achieved, the use amount of catalysts is less, the yield is up to 90%, the enantioselectivity can reach 99%, the reaction time is short, the enantioselectivity is good, and the method can be used for high-efficiency synthesis of the chiral benzodiazepine compound.
Owner:JILIN UNIV +1
Preparation method of deuterated amino acid ester compound
InactiveCN113214099AEasy to operateGentle operationIsotope introduction to heterocyclic compoundsOrganic compound preparationOrganic solventMedicinal chemistry
The invention discloses a method for efficiently preparing chiral deuterated amino acid ester, which is characterized in that in a common organic solvent, asymmetric transfer deuterization of imidic acid ester is realized by taking imidic acid ester as a raw material and taking deuterated methanol as a deuterium source and adopting transition metal catalysis. The target product namely chiral deuterated amino acid ester is obtained. The reaction general formula of the chiral deuterated amino acid ester is shown in the specification. The method for preparing the chiral deuterated amino acid ester by taking the deuterated methanol as the deuterium source comprises the steps of reaction system establishment, main reaction and post treatment. The preparation method of the deuterated chiral amino acid ester is simple to operate, mild, efficient, green and high in deuteration rate.
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA
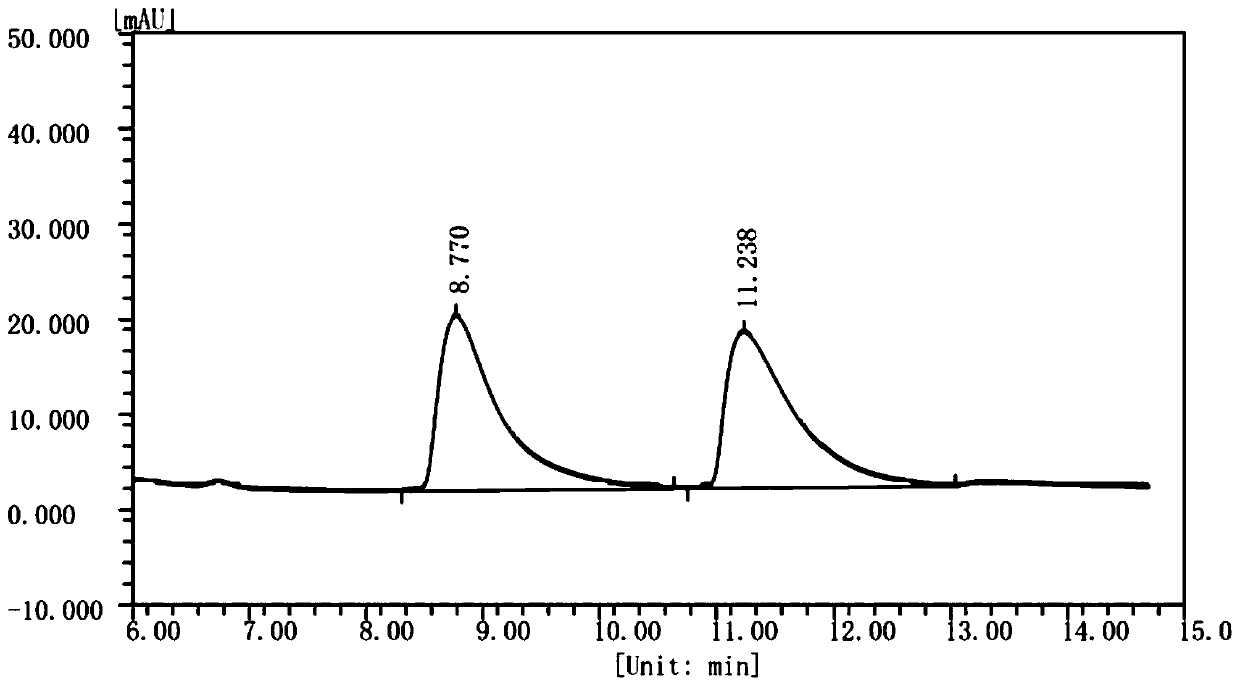
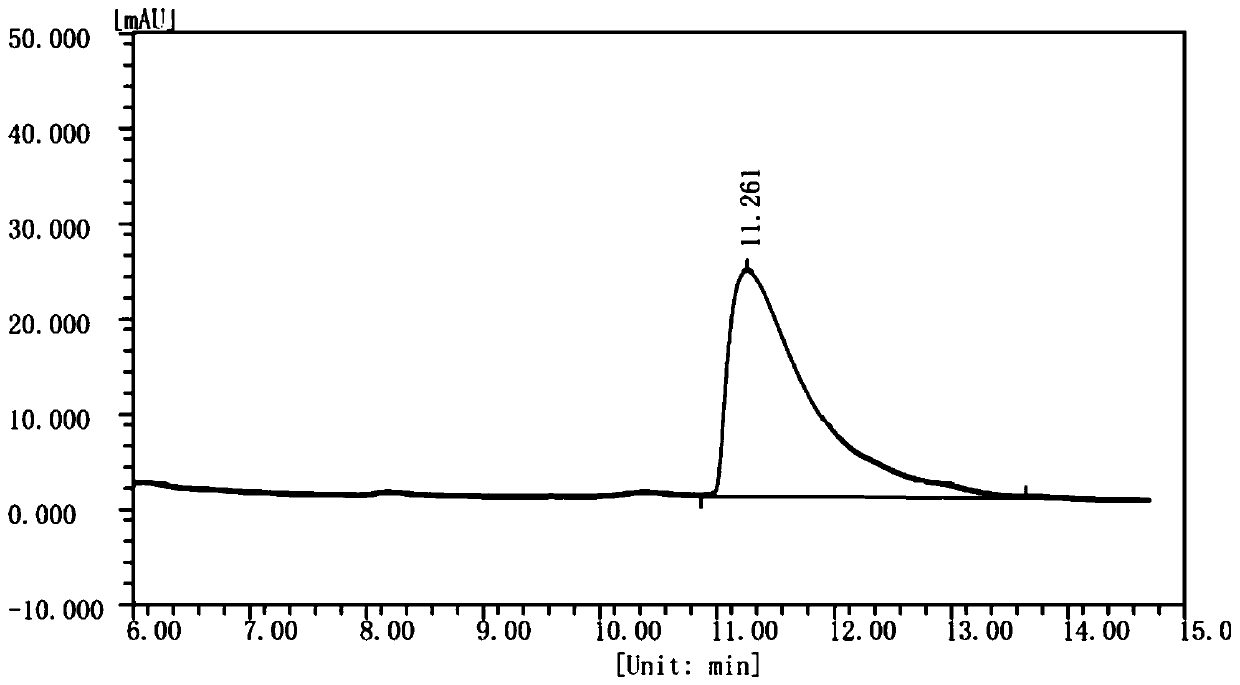
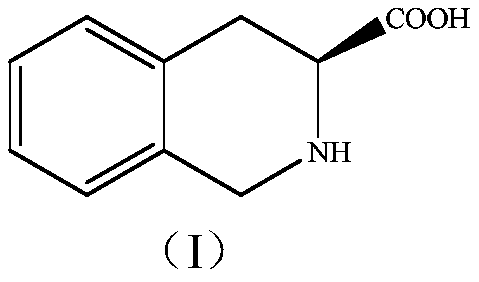
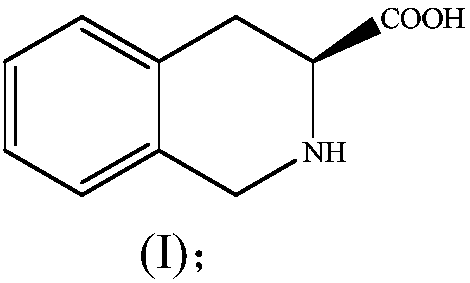
Method for preparing (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid through chemical enzyme process
ActiveCN111254181AHigh yieldImprove reaction efficiencyOrganic chemistryFermentationDehydrogenationCarboxylic acid
The invention discloses a method for preparing (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid through a chemical enzyme process. The method comprises the following steps: with 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid as a substrate, stereoselectively catalyzing an R-type isomer by utilizing D-amino acid oxidase; carrying out oxidative dehydrogenation to generate a corresponding imidic acid, wherein an S-type isomer which is not catalyzed is kept in a reaction system; reacting the imidic acid with an imidic acid reducing agent to generate a racemic substrate; and stereoselectivelycatalyzing the R-type isomer in the racemic substrate under the action of D-amino acid oxidase to prepare the S-type isomer. According to the method, the reaction yield can reach 80.6% or above, an ee value is larger than 99%, and the method has the advantages of mild reaction conditions, high stereoselectivity, high reaction efficiency, high yield, relatively simple process and the like.
Owner:TONGLI BIOMEDICAL +1
Method for preparing deuterated amino-acid ester by taking heavy water as deuterium source
InactiveCN111004076AEasy to operateGentle operationIsotope introduction to heterocyclic compoundsOrganic compound preparationArylPtru catalyst
The invention discloses a method for preparing deuterated amino-acid ester by taking heavy water as a deuterium source. According to the method, an imino acid ester compound and heavy water are takenas reaction raw materials, Lewis acid is taken as a catalyst, the target deuterated amino acid ester compound is synthesized through reaction in an organic solvent under the action of a reducing agent, and a reaction formula is shown in the specification, in the formula, R1 and R3 are aryl, and R2 is alkyl. The invention provides the preparation method of the deuterated amino-acid ester. The preparation method is simple to operate, is mild, efficient, and green, and has a high level of deuterium doping.
Owner:YUNNAN MINZU UNIV
Method for preparing (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid through multienzyme coupling
The invention discloses a method for preparing (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid through multienzyme coupling. The method comprises the following steps: by taking raceme of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid or raceme of 1,2,3,4-tetrahydroisoquinoline-3-formate as a substrate, enabling (R)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid in the substrate to react to generate imidic acid shown in a formula (II) under the catalysis action of oxidative dehydrogenase; and converting the imidic acid shown in the formula (II) into the (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid in the presence of piperidine acid reductase and a coenzyme capable of providing hydride ions. The method is gentle in reaction condition, good in stereoselectivity, high in reaction efficiency, high in conversion rate, and the like.
Owner:ZHEJIANG UNIV +1
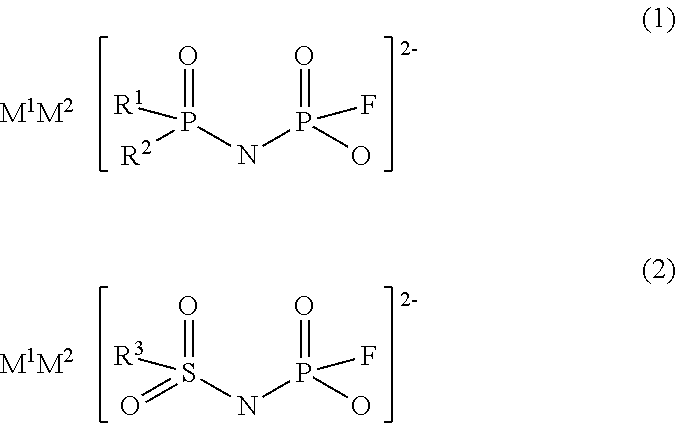
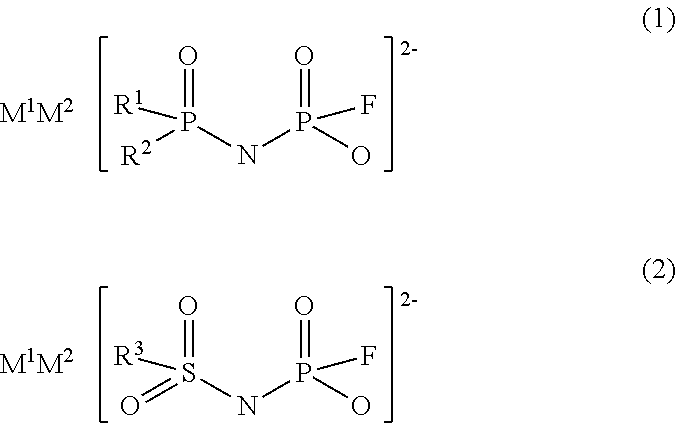
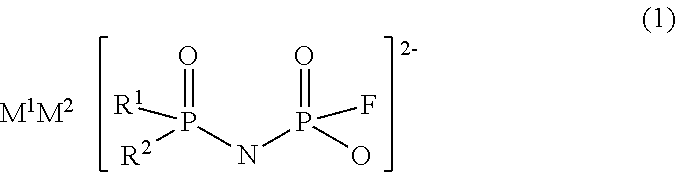
Imidic acid compound having divalent anion and process for producing the same
ActiveUS20170267528A1Low production costNitrosyl chlorideHybrid capacitor electrolytesAntistatic agentAlkoxy group
Provided is a novel imidic acid compound having a divalent anion useful as a pharmaceutical intermediate, an agrochemical intermediate, an acid catalyst, a battery electrolyte or an antistatic agent. The imidic acid compound is a divalent imidic acid compound represented by the following general formula (1) or (2).[In formulae (1) and (2), R1 to R3 represent a fluorine atom or an organic groups selected from a linear or branched C1-10 alkoxy group, a C2-10 alkenyloxy group, a C2-10 alkynyloxy group, a C3-10 cycloalkoxy group, a C3-10 cycloalkenyloxy group and a C6-10 aryloxy group, and wherein a fluorine atom, an oxygen atom or an unsaturated bond may also be present in the organic group. M1 and M2 represent protons, metal cations or onium cations.]
Owner:CENT GLASS CO LTD
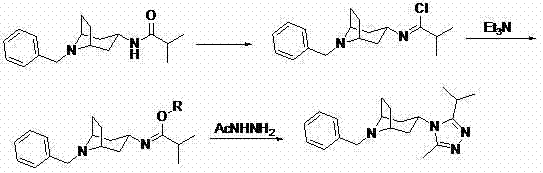
Preparation method for 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazole-4-base)-8-azabicyclo[3.2.1]octane
The invention discloses a preparation method for 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazole-4-base)-8-azabicyclo[3.2.1]octane. The preparation method comprises the following steps of: heating and refluxing N-(8-benzyl-8-azabicyclo[3.2.1]octyl-3-base)isobutyramide serving as a raw material and a vulcanization reagent in a non-proton solvent to obtain N-(8-benzyl-8-aza-bicyclo[3.2.1]octyl-3-base)isobutyl sulfamide; making sodium alcoholate react with halogenated hydrocarbon or sulfate to obtain N-(8-benzyl-8-aza-bicyclo[3.2.1]octyl-3-base)isobutyl imine acid thioester; heating and refluxing an alcohol with 2-5 carbon atoms and acethydrazide to obtain crude 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazole-4-base)-8-aza-bicyclo[3.2.1]octane; and refining normal heptane and ethyl acetate to obtain a high-purify target product. Due to the adoption of the method for preparing the 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazole-4-base)-8-azabicyclo[3.2.1]octane, water is prevented from interfering an intermediate reaction process, the yield is high, and the product purity is high.
Owner:ZHEJIANG UNIV +1
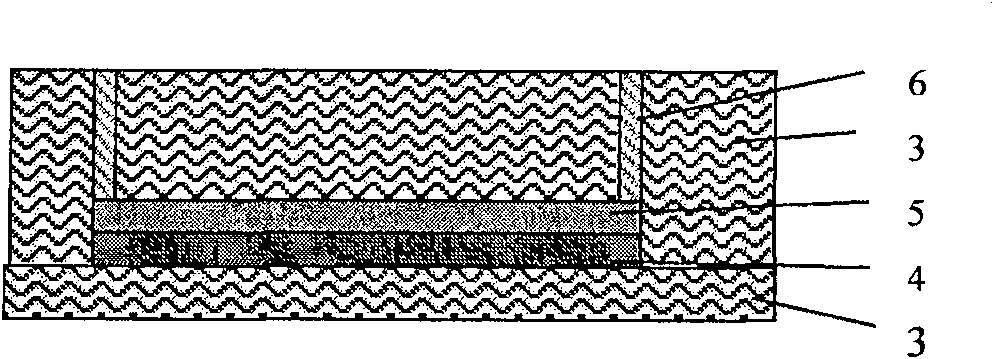
Preparation method of biological microelectrode array based on flexible substrate
InactiveCN100551462CSimple processImprove yieldEar treatmentDecorative surface effectsMeasuring instrumentMicroelectrode
Owner:SHANGHAI JIAO TONG UNIV
Preparation method of 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo (3.2.1)octane
The invention provides a preparation method of 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)octane, which comprises the following steps successively: taking N-(8-benzyl-8-azabicyclo(3.2.1)oct-3-yl)-isobutyramide as a raw material and heating to reflux with a chlorinating agent in an aprotic solvent, then reacting with alcohol under the action of triethylamine to obtain isobutyl-N-(8-benzyl-8-azabicyclo(3.2.1)oct-3-yl)imidate, and heating to reflux with acetohydrazide in alcohol of C2-C5 to obtain 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo [3.2.1] octane crude, and finally purifying by n-heptane and ethyl acetate to obtain the target product with high purity. The 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo [3.2.1] octane prepared by the method provided by the invention has the advantages of less byproducts, high yield and high product purity.
Owner:WENZHOU MEDICAL UNIV
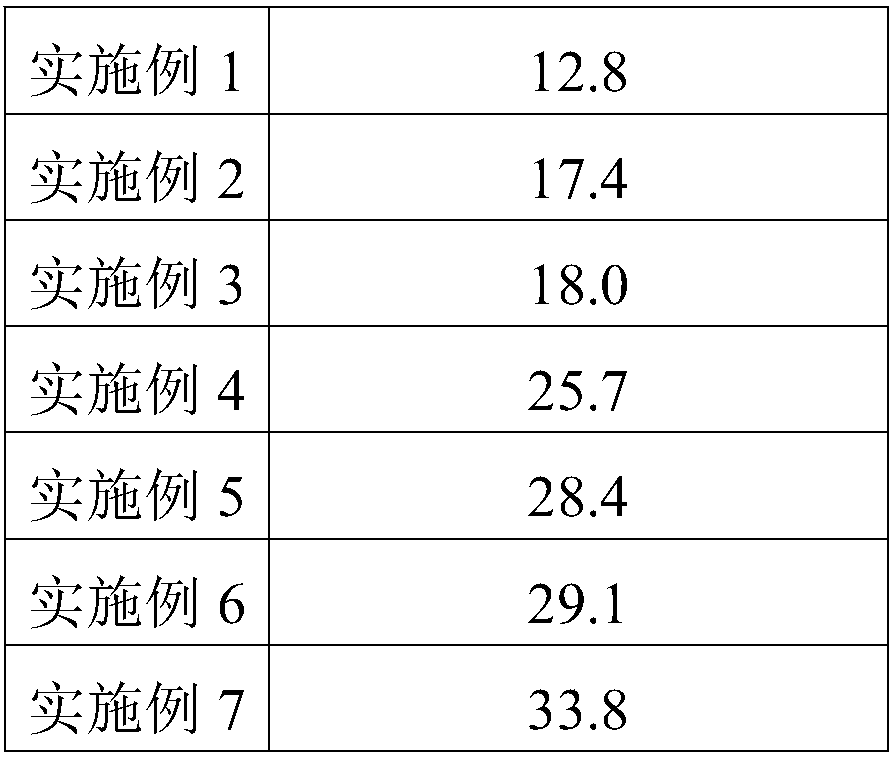
Selenium-enriched ampelopsis grossedentata and preparation method thereof
The invention provides selenium-enriched ampelopsis grossedentata and a preparation method thereof and belongs to the field of tea preparation. The defect of excessive loss of nutrients in a traditional ampelopsis grossedentata production process is overcome. The preparation method of the selenium-enriched ampelopsis grossedentata comprises the following steps of pretreatment, wherein freshly collected ampelopsis grossedentata leaves are soaked in an ethanol solution of alpha-imidic acid for 12-36 hours, and solids are taken out and dried; killing out; fermentation; evaporation of water. In addition, a flavoring step can also be included. The selenium-enriched ampelopsis grossedentata has not only high selenium content but also abundant dihydromyricetin, and the nutritional value of the selenium-enriched ampelopsis grossedentata serving as a beverage is greatly improved. The total selenium content is as high as 2.67 mg / kg, and the highest content of dihydromyricetin can also reach 33.8%.
Owner:滁州市恩典硒文化传媒有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
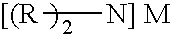

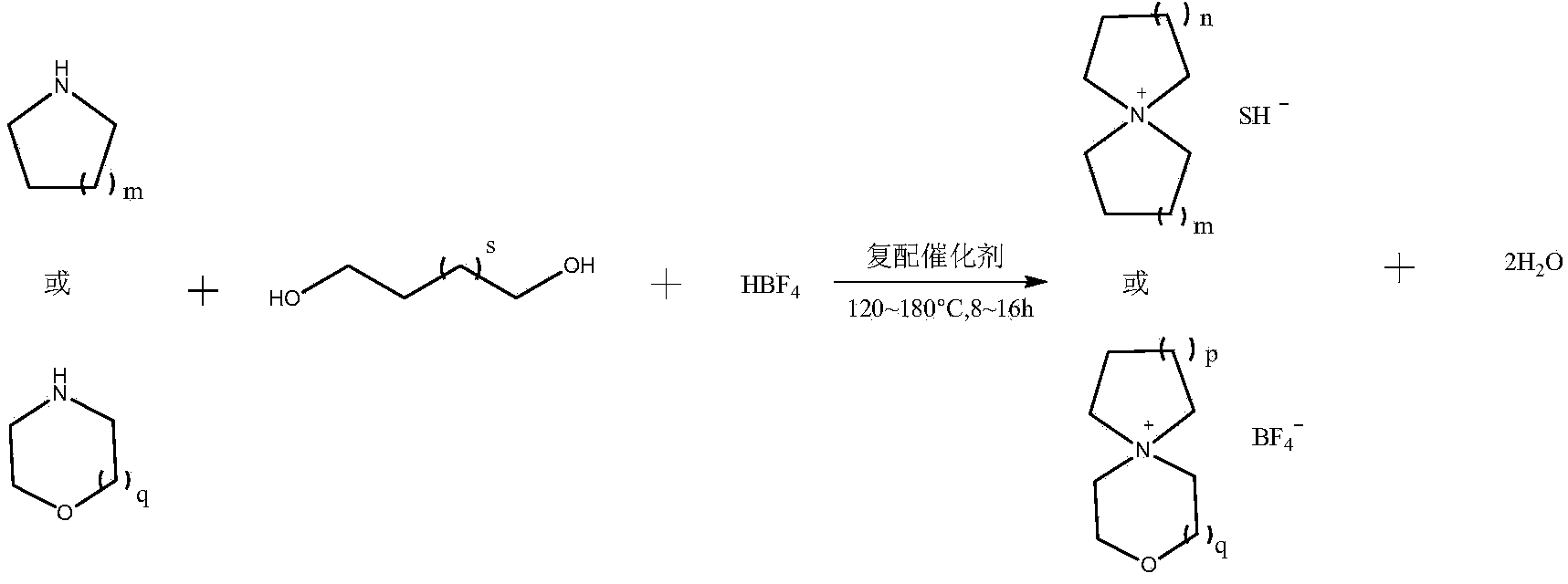
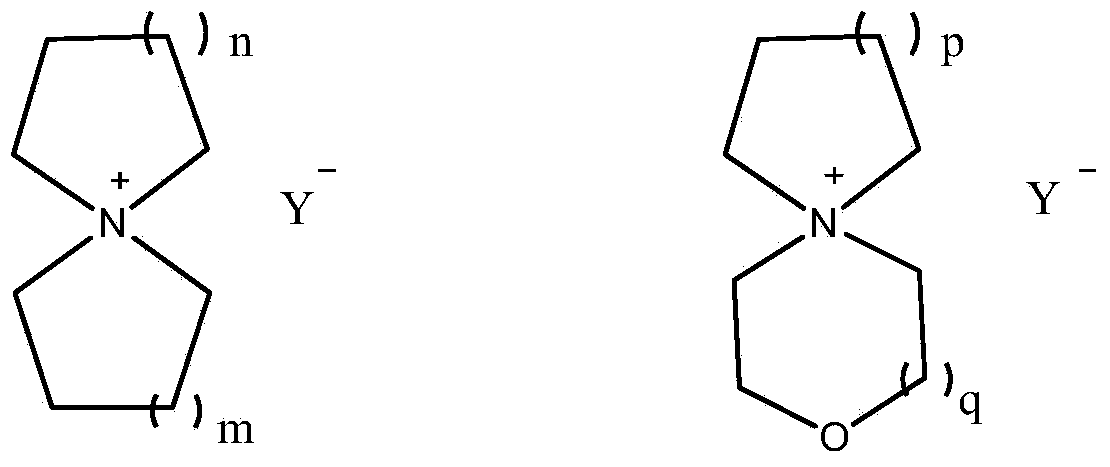
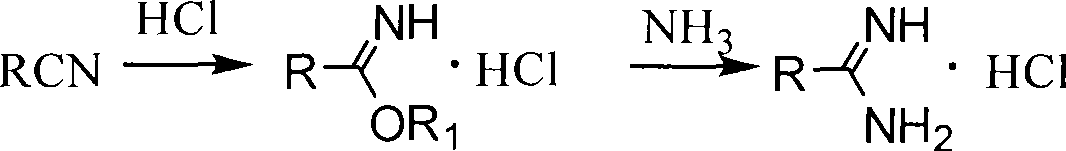









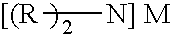



![Preparation method for 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazole-4-base)-8-azabicyclo[3.2.1]octane Preparation method for 8-benzyl-3-(3-isopropyl-5-methyl-4H-1,2,4-triazole-4-base)-8-azabicyclo[3.2.1]octane](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/23f8df69-3735-44e5-a45f-2fca4da141ee/606880DEST_PATH_IMAGE001.PNG)