3-pyridyl-3-amino-2-cyanoacrylate compound, and preparation method and application thereof
A technology of cyanoacrylate and compound, which is applied in the field of 3-pyridyl-3-amino-2-cyanoacrylate compound and its preparation, can solve the problem of narrow action spectrum of cyanostrobin and the use of dosage is not very high. Efficiency and other issues, to achieve the effect of outstanding prevention and control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
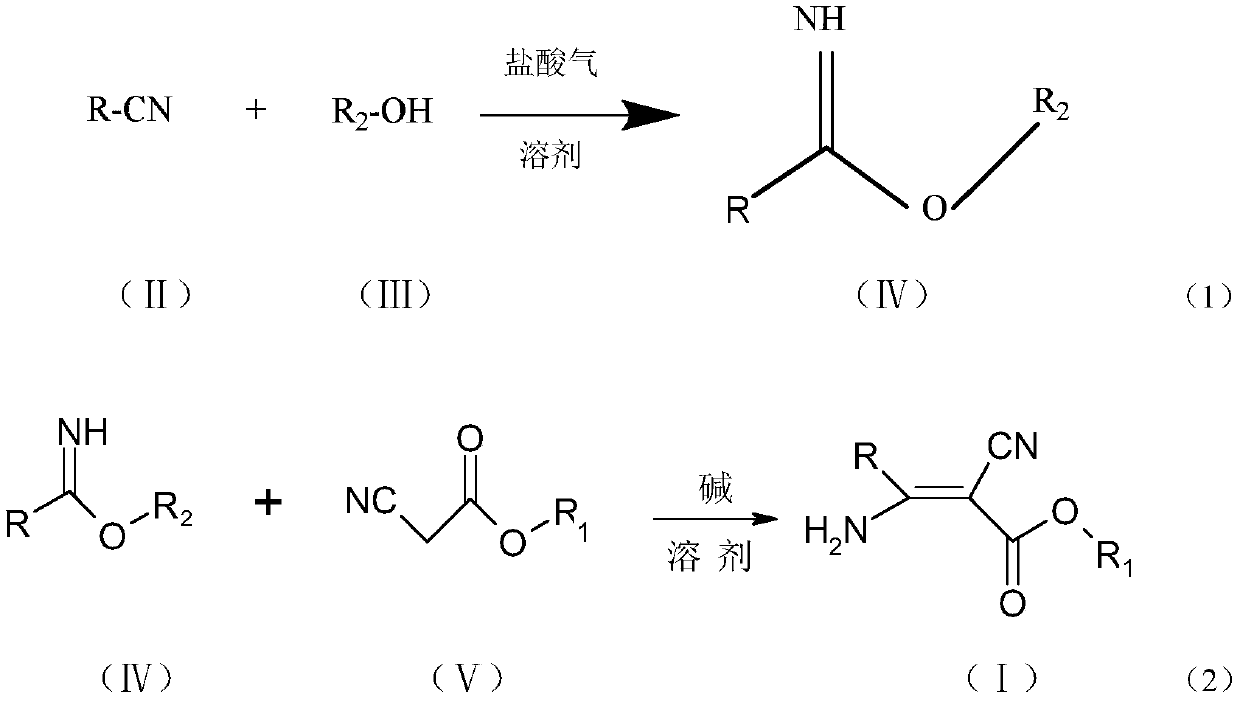
[0058] The synthetic route of general formula (1) compound is:
[0059]
[0060]
[0061] Compound NO.001 (R=pyridin-4-yl, R 1 = ethyl, R 2 =ethyl): Synthesis of ethyl 2-cyano-3-amino-3-(pyridin-4-yl)acrylate
[0062] Add 5.0g (48mmol) of 4-cyanopyridine and 2.2g (48mmol) of absolute ethanol into 20ml of dichloromethane, cool down to 0°C, and pass in 5.26g (144mmol) of hydrochloric acid gas until the reaction solution is clarified, and keep it warm for the reaction until the raw materials are consumed. Add 10% sodium carbonate aqueous solution dropwise under stirring until pH = 7-8, let stand to separate the oil layer, and remove the solvent to obtain 7.7 g of ethyl pyridine-4-carboximidate, content: 92.0%, yield: 98.4% .
[0063] Add 8.0g (53mmol) of ethyl pyridine-4-carboximidate and 0.3g (3mmol) of triethylamine into 40ml of absolute ethanol, heat to reflux at about 78°C, and dropwise add 6.0g (53mmol) of ethyl cyanoacetate Esters, after dropping the heat preserv...
preparation Embodiment 1
[0076] Formulation Example 1: emulsion in water
[0077] 20 parts of the compound of the present invention, 12 parts of toluene, 6 parts of ethylene oxide-propylene oxide block copolymer, 6 parts of xanthan gum, 4.5 parts of ethylene glycol antifreeze, 4 parts of propylene glycol antifreeze, and 0.8 parts of organic silicon , 46.7 parts of water, obtain the water emulsion that active ingredient is 20% by water emulsion processing technology.
preparation Embodiment 2
[0078] Formulation Example 2: Suspending agent:
[0079]25 parts of the compound of the present invention, 6 parts of wetting agent sodium p-methyl fatty amidobenzene sulfonate, 2 parts of dispersant alkylphenol polyoxyethylene ether formaldehyde condensate, 6 parts of thickener carboxymethylcellulose sodium, 1 part of preservative sodium salicylate, 2 parts of antifreeze agent propylene glycol, 1 part of antifoaming agent silicone oil, 57 parts of water, according to the suspending agent processing technology to obtain a suspending agent with an active ingredient of 25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com