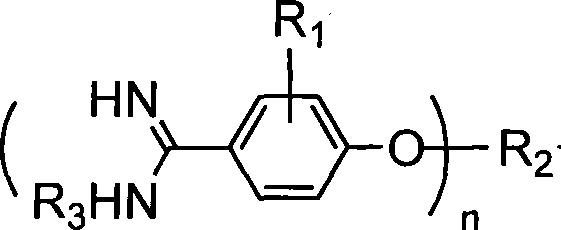
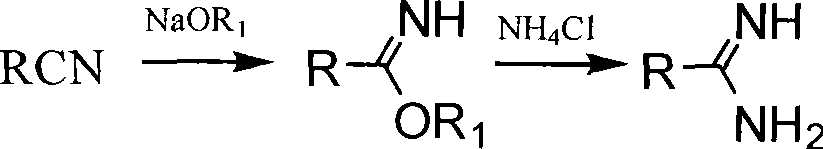
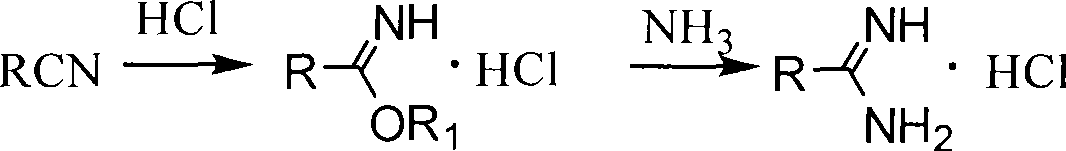Method for synthesizing alkoxy aromatic amidine compounds
A technology of alkoxy aromatic amidines and alkoxy aromatic amidines hydrochloride, which is applied in the field of synthesis of aromatic amidines, can solve the problems of cumbersome post-processing operations, large losses, and difficulties in solvent recovery, and saves the intermediate treatment process , Improve product yield and reduce solvent loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Dissolve 24g of sodium metal in 500mL of anhydrous methanol, after cooling slightly, add 32g of p-cyanophenol and 76.9mL of dibromohexane, heat to reflux for 16h, cool to 0°C, and pass dry hydrogen chloride gas into the reaction solution to saturation , close the reactor, control the temperature at 30-40°C, react for 3 days, pass dry nitrogen gas, until the acid gas is less, cool to 0°C, pass dry ammonia gas, heat to 60°C, react for 6h , cooled to room temperature, suction filtered, the filter cake was added to water, stirred to form a suspension, solid potassium carbonate was added, stirred for 30 min, suction filtered, washed with water, and dried to obtain 28.5 g of a white powdery solid.
Embodiment 2
[0067] The operation was the same as in Example 1, and the solvent was absolute ethanol to obtain 29.6 g of white powdery solid.
Embodiment 3
[0069] The operation is the same as that in Example 2, and the ammonia gas is changed to add 50 mL of methylamino alcohol solution (30%), heated to 60 ° C, reacted for 4 hours, cooled to room temperature, filtered with suction, the filter cake is added to water, stirred into a suspension, and solid carbonic acid is added Potassium, stirred for 30 minutes, suction filtered, washed with water, and dried to obtain 26.7 g of white powdery solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com