Preparation method of deuterated amino acid ester compound
A technology for ester compounds and amino acid esters, which is applied in the field of preparation of chiral deuterated amino acid esters, can solve the problems of complex synthesis conditions, harshness, inability to meet application value and market value, etc., and achieves the effect of simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
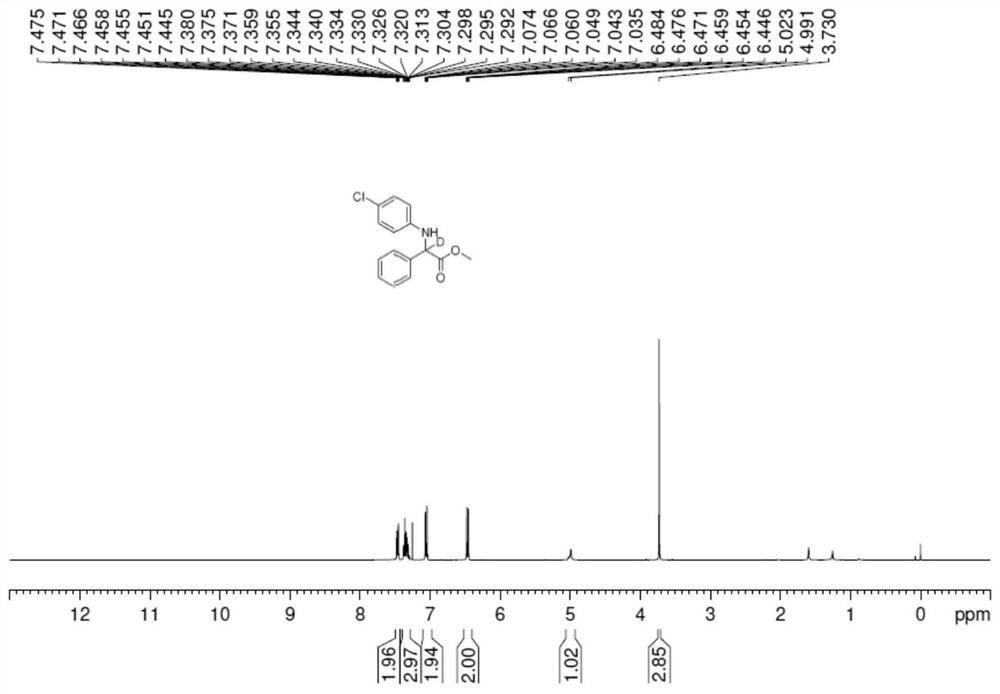
[0027] In an anhydrous and oxygen-free glove box under argon atmosphere, (R)-SEGPHOS (0.012mmol)Pd(OAc) 2 (0.005mmol) into the reaction tube in turn, then add 1ml 1,2-dichloroethane ring and stir for 30min, then add Zn(OTf) 2 (Zinc trifluoromethanesulfonate) (0.02mmol) was stirred for 10min, and then 0.2mmol of imidate derivative, deuterated methanol (1.0mmol), and 1ml of 1,2-dichloroethane were added. React in an oil bath at 70°C, monitor and detect the reaction by TLC, concentrate after the completion of the reaction, and pass through a column with silica gel to obtain a white solid with a yield of 89% and an ee value of 87%. The income rate is 97%.
[0028] 1 H NMR (400MHz, CDCl 3 ):δ7.49-7.44(m,2H),7.38-7.29(m,3H),6.74-6.71(m,2H),6.55-6.51(m,2H),5.01(s,0.03H),4.66( s, 1H), 3.71 (d, J=7.16Hz, 6H).
Embodiment 2
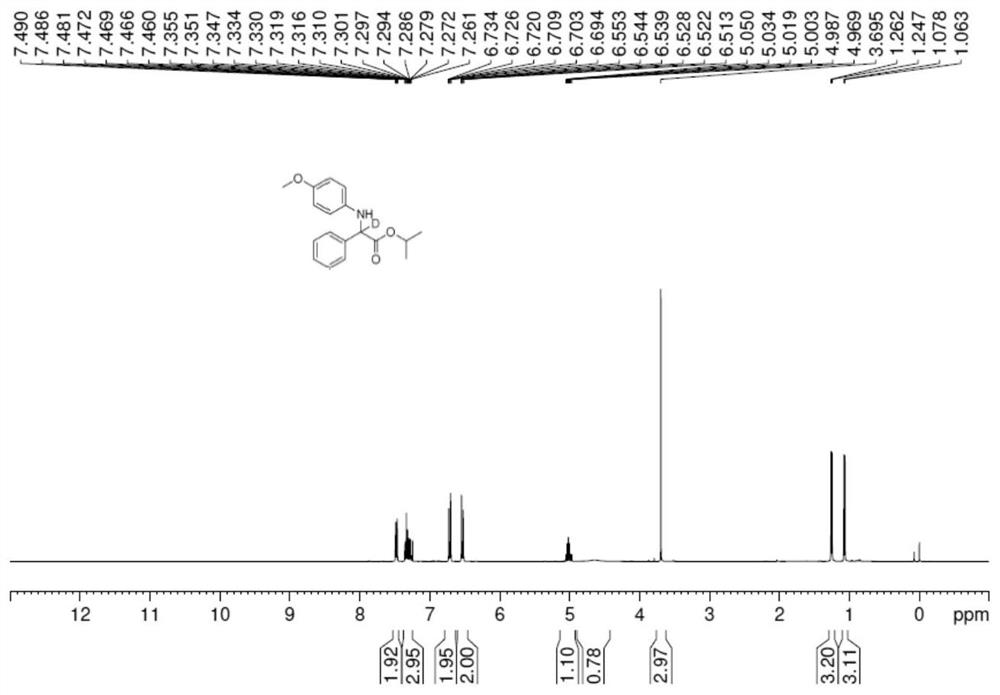
[0030]
[0031] In an anhydrous and oxygen-free glove box under argon atmosphere, (R)-SEGPHOS (0.012mmol)Pd(OAc) 2 (0.005mmol) into the reaction tube in turn, then add 1ml 1,2-dichloroethane ring and stir for 30min, then add Zn(OTf) 2 (Zinc trifluoromethanesulfonate) (0.02 mmol) was stirred for 10 min, and then 0.2 mmol of imidate derivatives, deuterated methanol (1.0 mmol), and 1 ml of 1,2-dichloroethane were added. React in an oil bath at 70°C, monitor and detect the reaction by TLC, concentrate after the completion of the reaction, and pass through a column with silica gel to obtain a white solid with a yield of 92% and an ee value of 88%. The income rate is 78%. 1 H NMR (400MHz, DMSO-d 6 )δ8.33 (s, 1H), 7.63–6.97 (m, 5H), 4.45 (dd, J=9.3, 7.8Hz, 0.21H), 3.59 (s, 3H), 3.00 (d, J=13.7Hz, 0.24H), 2.87(d,J=13.2Hz,1H), 1.79(s,3H).
Embodiment 3
[0033]
[0034] In an anhydrous and oxygen-free glove box under argon atmosphere, (R)-SEGPHOS (0.012mmol)Pd(OAc) 2 (0.005mmol) into the reaction tube in turn, then add 1ml 1,2-dichloroethane ring and stir for 30min, then add Zn(OTf) 2 (Zinc trifluoromethanesulfonate) (0.02 mmol) was stirred for 10 min, and then 0.2 mmol of imidate derivatives, deuterated methanol (1.0 mmol), and 1 ml of 1,2-dichloroethane were added. React in an oil bath at 70°C, TLC monitors and detects the reaction. After the reaction is completed, concentrate and pass through a column with silica gel to obtain a white solid with a yield of 95% and an ee value of 90%. The income rate is 78%. 1H NMR (400MHz, Chloroform-d) δ8.33 (d, J = 7.8Hz, 1H), 7.19 (td, J = 7.5, 1.0Hz, 1H), 6.78 (dt, J = 9.0, 1.4Hz, 3H) ,4.44(ddd,J=9.4,7.7,5.4Hz,022H),3.72(s,3H),3.60(s,3H),2.98(dd,J=13.7,5.5Hz,0.20H),2.84(dd, J=13.7,9.4Hz,1H),1.79(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com