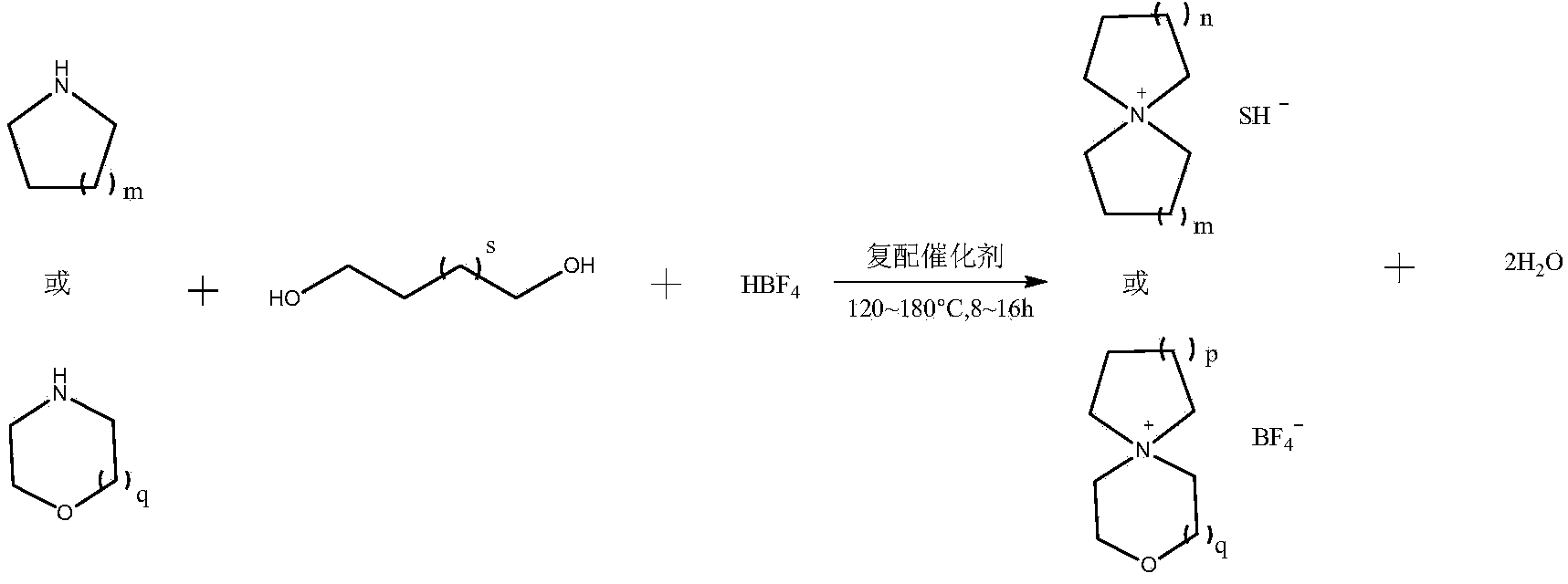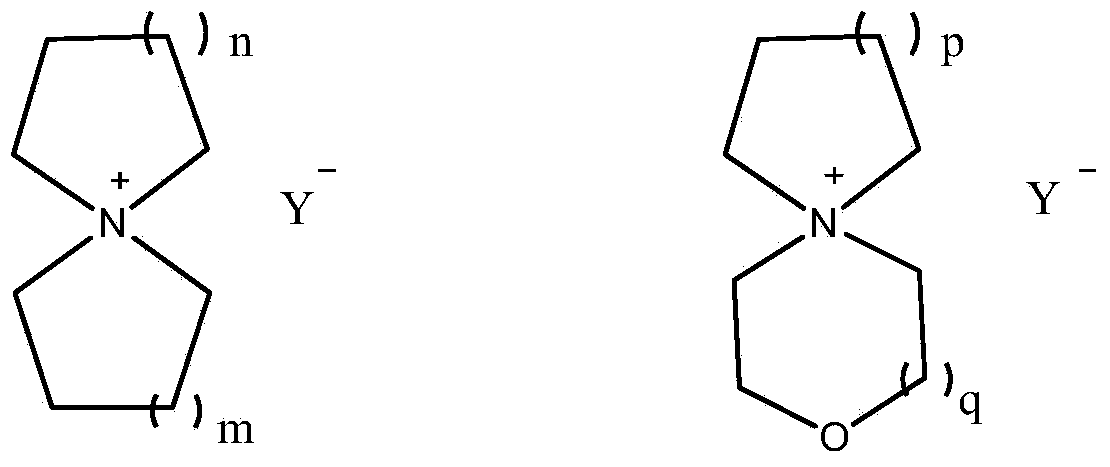Preparation method of spiro-quaternary ammonium salt electrolyte
A technology of spirocyclic quaternary ammonium salts and electrolytes, which is applied in hybrid capacitor electrolytes, organic chemistry, etc., can solve the problems of difficulty in meeting the requirements of electrochemical products, large residual halogen ions, and low synthesis yields, and achieve product yields High, high purity, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
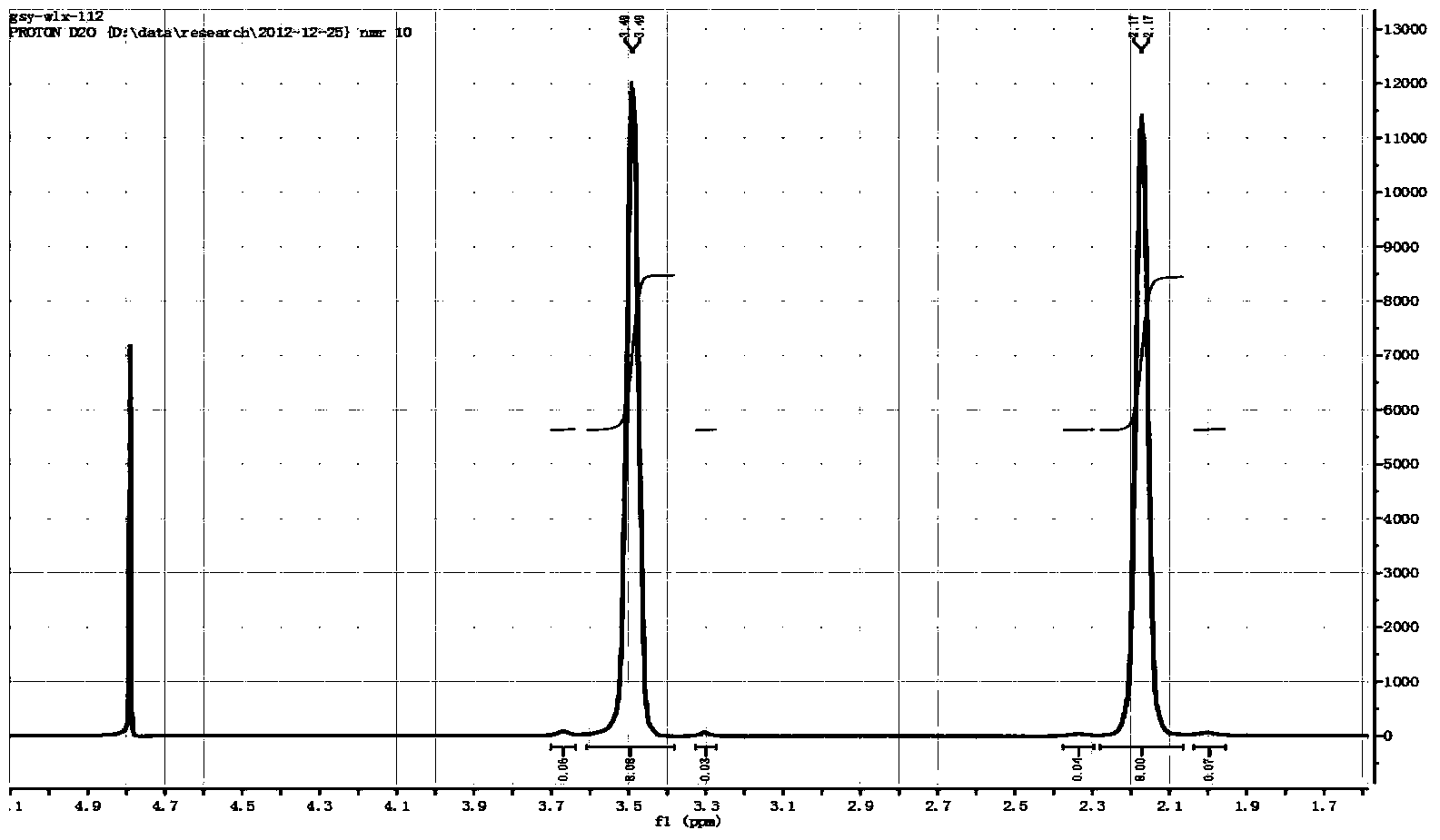
[0030] Embodiment 1: Preparation of tetrafluoroborate spiro 1,1'-bispyrrole quaternary ammonium salt
[0031] In a 2L tetrafluoro autoclave, add inert solvent acetonitrile (328g, 8mol), tetrahydropyrrole (71g, 1mol), 1,4 butanediol (75g, 0.83mol), composite catalyst (0.046 g deoxidation type palladium catalyst; 1.375g styrene series strongly acidic cation exchange resin), then slowly add 45% concentration of tetrafluoroboric acid aqueous solution (179g, containing tetrafluoroboric acid 81g), be warming up to 150 ℃ of reaction 8 hours. The above reaction solution was lowered to room temperature, filtered under reduced pressure, and concentrated to a semi-solid state under vacuum at 120°C. After the concentrated solution was cooled to 60°C, an appropriate amount of ethanol was added to produce a large amount of white crystals, and the crude product of spiro 1,1'-bispyrrole quaternary ammonium tetrafluoroborate was obtained by filtration, which was then recrystallized and dried w...
Embodiment 2
[0036] Embodiment 2: Preparation of spiropyrrole-1,1'-piperidine quaternary ammonium tetrafluoroborate
[0037] In a 2L tetrafluoro autoclave, add inert solvent acetonitrile (328g, 8mol), hexahydropyridine (85g, 1mol), 1,4 butanediol (75g, 0.83mol), composite catalyst (0.055 g deoxidation type palladium catalyst; 1.65g styrene series strongly acidic cation exchange resin), then slowly add 45% concentration of tetrafluoroboric acid aqueous solution (179g, containing tetrafluoroboric acid 81g), be warming up to 150 ℃ of reaction 8 hours. The above reaction solution was lowered to room temperature, filtered under reduced pressure, and concentrated to a semi-solid state under vacuum at 120°C. After the concentrated solution was cooled to 60°C, an appropriate amount of ethanol was added to produce a large amount of white crystals, and the crude product of spiropyrrole-1,1'-piperidine quaternary ammonium tetrafluoroborate was obtained by filtration, and then recrystallized and dried...
Embodiment 3
[0041] Embodiment 3: Preparation of tetrafluoroborate spiro 1,1'-bispiperidine quaternary ammonium salt
[0042] In the 2L tetrafluoro autoclave, add inert solvent acetonitrile (328g, 8mol) successively under stirring state, hexahydropyridine (85g, 1mol), 1,5 pentanediols (86.5g, 0.83mol), composite catalyst ( 0.055g deoxidation type palladium catalyst; 1.65g styrene series strongly acidic cation exchange resin), then slowly add 45% concentration of tetrafluoroboric acid aqueous solution (179g, containing tetrafluoroboric acid 81g), be warming up to 155 ℃ and react for 8 hours. The above reaction solution was lowered to room temperature, filtered under reduced pressure, and concentrated to a semi-solid state under vacuum at 120°C. After the concentrated solution was cooled to 60°C, an appropriate amount of ethanol was added to produce a large amount of white crystals, and the crude product of spiro 1,1'-bispiperidine quaternary ammonium tetrafluoroborate was obtained by filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com