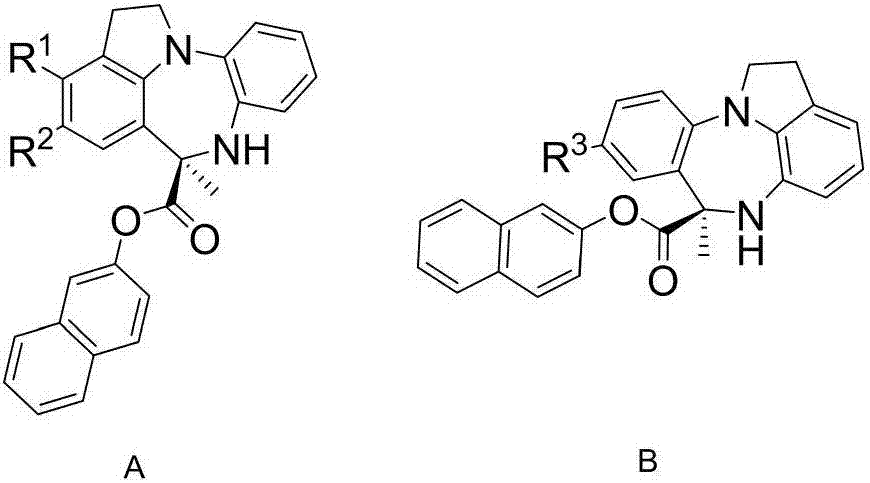
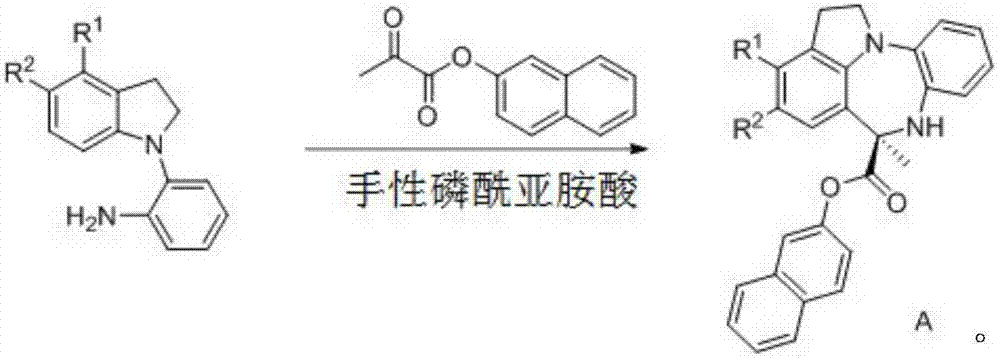
Chiral benzodiazepine compound and synthesis method thereof
A synthesis method and benzodiazepine technology are applied in the field of chiral benzodiazepine compounds and their synthesis, and achieve the effects of good application prospect, reduced production cost and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Preparation of chiral benzodiazepines
[0034] Dissolve 2-(1H-indoline)aniline (21g, 0.1mol) and pyruvate-2-naphthyl ester (21g, 0.1mol) in 1L tetrahydrofuran, and add 1-naphthyl at the 3,3' position while stirring The H8-BINOL derived phosphoramidite acid (0.2g) and Molecular sieves (70g) were reacted at 30°C for 48 hours. After the reaction was complete, the product was purified by column chromatography to obtain a white solid (36.5g, 93%, 95%ee). 95%ee[Daicel Chiralcel AD-H column, n-hexane / i-PrOH=70:30, 1.0mL / min, λ=254nm, t(minor)=37.20min, t(major)=21.58min]; 1 H NMR (300MHz, DMSO-d 6 )δ7.90–7.71(m,3H),7.54–7.43(m,2H),7.19–6.99(m,5H),6.94–6.79(m,2H),6.73–6.58(m,2H),5.92( s, 1H), 4.12–3.96 (m, 1H), 3.84 (dd, J=16.3, 7.1 Hz, 1H), 3.19–3.02 (m, 2H), 2.00 (s, 3H).
Embodiment 2
[0035] Embodiment 2: Preparation of chiral benzodiazepines
[0036] Dissolve 2-(1H-indoline)aniline (6.3g, 30mmol) and pyruvate-2-naphthyl ester (6.3g, 30mmol) in 200mL ether, and add 1-naphthyl at the 3,3' position while stirring The H8-BINOL derived phosphoramidite acid (0.2g) and Molecular sieves (80g) were reacted at 30°C for 48 hours. After the reaction was complete, the product was purified by column chromatography to obtain a white solid (10.7g, 91%, 95%ee). 95%ee[Daicel Chiralcel AD-H column, n-hexane / i-PrOH=70:30, 1.0mL / min, λ=254nm, t(minor)=37.20min, t(major)=21.58min]; 1 H NMR (300MHz, DMSO-d 6 )δ7.90–7.71(m,3H),7.54–7.43(m,2H),7.19–6.99(m,5H),6.94–6.79(m,2H),6.73–6.58(m,2H),5.92( s, 1H), 4.12–3.96 (m, 1H), 3.84 (dd, J=16.3, 7.1 Hz, 1H), 3.19–3.02 (m, 2H), 2.00 (s, 3H).
Embodiment 3
[0037] Embodiment 3: Preparation of chiral benzodiazepines
[0038]Dissolve 2-(1H-indoline)aniline (10.5g, 50mmol) and 2-naphthyl pyruvate (13.2g, 60mmol) in 500mL tetrahydrofuran, and add 2-naphthyl at the 3,3' position while stirring The H8-BINOL derived phosphoramidite acid (0.4g) and Molecular sieves (35g) were reacted at 30°C for 48 hours. After the reaction was complete, the product was purified to obtain a white solid (16.5g, 90%, 95%ee). 95%ee[Daicel Chiralcel AD-H column, n-hexane / i-PrOH=70:30, 1.0mL / min, λ=254nm, t(minor)=37.20min, t(major)=21.58min]; 1 H NMR (300MHz, DMSO-d 6 )δ7.90–7.71(m,3H),7.54–7.43(m,2H),7.19–6.99(m,5H),6.94–6.79(m,2H),6.73–6.58(m,2H),5.92( s, 1H), 4.12–3.96 (m, 1H), 3.84 (dd, J=16.3, 7.1 Hz, 1H), 3.19–3.02 (m, 2H), 2.00 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com