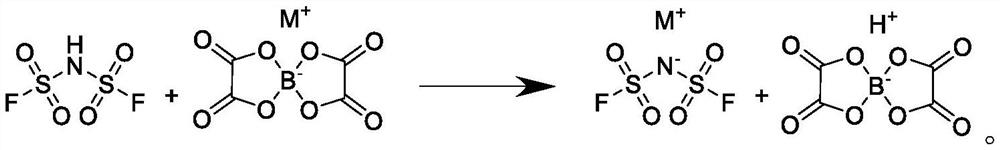
Preparation method of bis(fluorosulfonyl)imide salt
The technology of bisfluorosulfonimide salt and bisfluorosulfonimide acid is applied in the field of preparation of bisfluorosulfonimide salt, and can solve the problems of difficult to meet the index, content of finished product, acid value, chromaticity difficult to meet the standard, The problems of chromaticity and turbidity increase, to achieve the effect of low halogen ion residue, low chromaticity and acid value, and low turbidity of insoluble matter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] First, 200 grams (1.036 moles) of lithium bisoxalate borate and 100 grams of ethyl acetate were mixed and dissolved in a drop tank, and then 178.4 grams (0.986 moles) of bisfluorosulfonylimidic acid and 1,1 , 800 grams of 2,2-tetrachloroethylene, when the temperature in the reaction vessel drops to -5°C, start to drop the ethyl acetate solution of lithium bisoxalate borate, keep the reaction for 3 hours after the addition, then filter the reaction solution, the filter cake Transferred to a desiccator, dried under reduced pressure to obtain 171.4 grams of bisfluorosulfonimide lithium salt, yield 93%, content 99.91%, acid value 20ppm, chromaticity 8 Hazen, 10% aqueous solution turbidity 2mg / L , turbidity of 10% dimethyl carbonate solution is 2mg / L, chloride ion 2.8ppm, fluoride ion 15ppm, SO4 2- Ion 0.8ppm.
Embodiment 2
[0026] First, 200 grams (0.953 moles) of sodium bisoxalate borate and 100 grams of ethyl propyl ether are mixed and stirred in a dripping tank to dissolve, and then 161 grams (0.890 moles) of bisfluorosulfonylimidic acid and 1 , 900 grams of 1,2-trichloroethylene, when the temperature in the reaction vessel drops to -10°C, start to add the ethyl propyl ether solution of sodium bisoxalate borate dropwise, keep warm for 5 hours after the dropwise addition, and then filter the reaction solution , the filter cake was transferred to a desiccator, and after drying under reduced pressure, 153.1 grams of bisfluorosulfonimide sodium salt was obtained, with a yield of 92%, a content of 99.90%, an acid value of 22 ppm, a chromaticity of 9 Hazen, and a 10% aqueous solution with a turbidity of 3mg / L, turbidity of 10% dimethyl carbonate solution is 3mg / L, chloride ion 2.1ppm, fluoride ion 14ppm, SO4 2- Ion 0.8ppm.
Embodiment 3
[0028] First, mix and dissolve 200 grams (0.885 moles) of potassium bisoxalate borate and 200 grams tetrahydrofuran in a drop tank, then mix 145.5 grams (0.804 moles) of bisfluorosulfonylimidic acid and 1,1,2 , 850 grams of 2-tetrachloroethane, when the temperature in the reaction vessel dropped to -15°C, the tetrahydrofuran solution of potassium bisoxalate borate was added dropwise. In the desiccator, 136.8 grams of difluorosulfonimide potassium salt was obtained after drying under reduced pressure, with a yield of 91%, a content of 99.91%, an acid value of 23 ppm, a chromaticity of 10 Hazen, and a turbidity of 10% aqueous solution of 2 mg / L, 10 The turbidity of % dimethyl carbonate solution is 3mg / L, the chloride ion is 2.5ppm, the fluoride ion is 12ppm, SO4 2- Ion 0.9ppm.
[0029] The following are comparative examples to illustrate the comparative advantages of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com