Production process of imidazole aldehyde
A production process and imidazolaldehyde technology are applied in the production technology field of imidazolyl, can solve the problems of long cycle, high cost, unfavorable promotion and industrialized production, etc., achieve good stability, reduce by-products, optimize reaction conditions and feed ratio Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
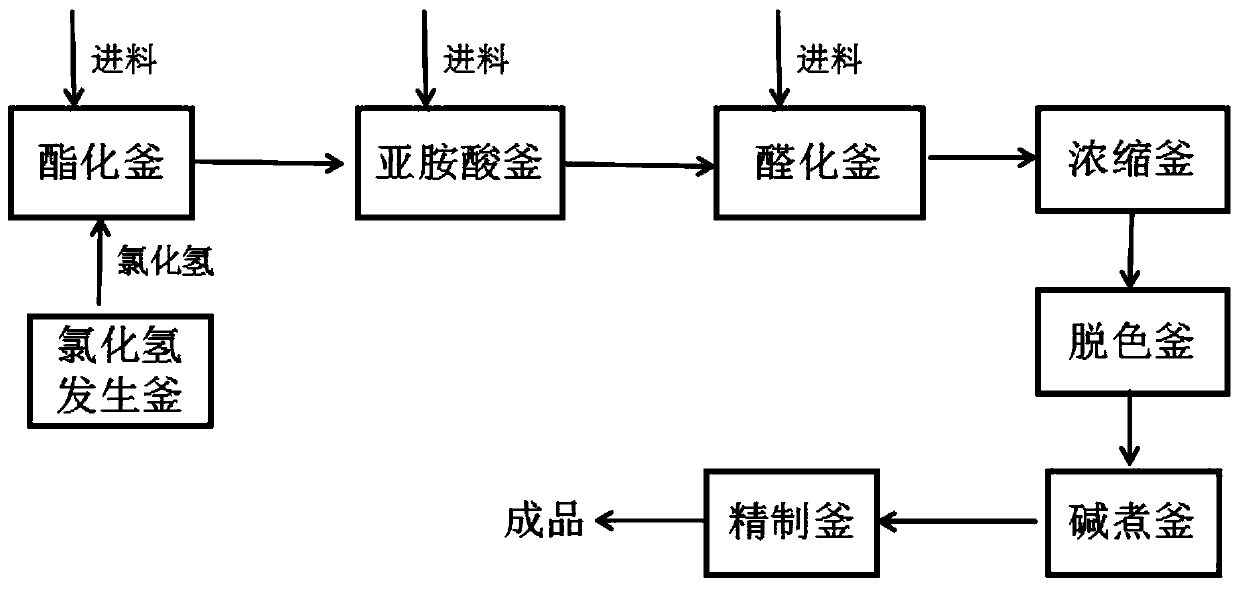
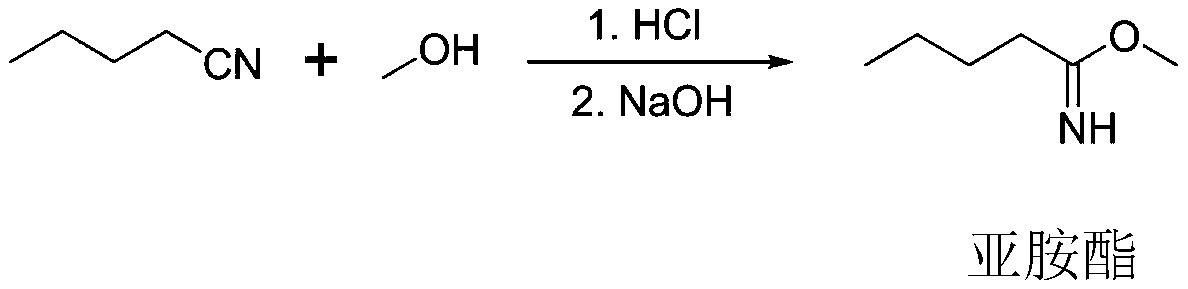
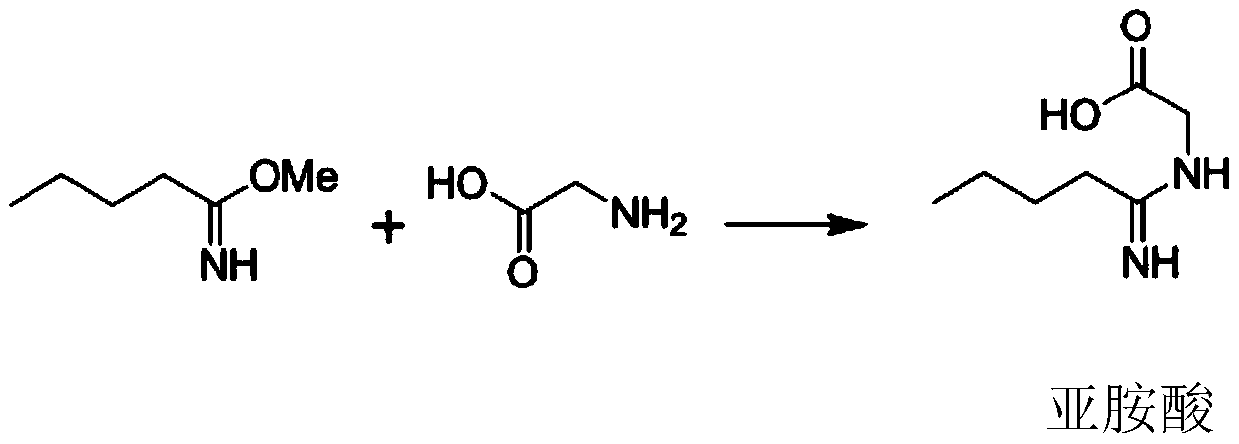
[0049] Put 500kg of valeronitrile and 300kg of methanol into the esterification kettle, then cool down to 0°C, and feed 280kg of hydrogen chloride gas (prepared by mixing phosphorus trichloride and hydrochloric acid solution according to the mass ratio of 2.0:1.65, and control the pressure in the kettle to be lower than 0.1MPa, The temperature is 7-9°C for reaction), after the aeration, keep the temperature at 10°C for 15h, add 200L of sodium hydroxide solution with a mass fraction of 30%, control the temperature below 10°C for 5h to obtain imidate, add 230kg of toluene, Extract to obtain imidate toluene solution; add 250kg of glycine, 750kg of methanol, control the temperature at 20°C for 3h, control the temperature below 55°C and the pressure at -0.01Mpa for vacuum distillation, and then carry out vacuum distillation at 55°C and -0.01MPa Bake for 3 hours to obtain imidic acid; put it into the hydroformylation kettle, add 550kg of phosphorus oxychloride, 200kg of toluene, cont...
Embodiment 2
[0052] Put 500kg of valeronitrile and 270kg of methanol into the esterification kettle, then cool down to 5°C, and feed 280kg of hydrogen chloride gas (prepared by mixing phosphorus trichloride and hydrochloric acid solution according to the mass ratio of 2.0:1.65, and control the pressure in the kettle to be lower than 0.1MPa, The temperature is 7-9°C for reaction), after the aeration, keep the temperature at 12°C for 12h, add 250L of sodium hydroxide solution with a mass fraction of 30%, control the temperature below 10°C for 8h to obtain imidate, add 250kg of toluene, Extract to obtain imidate toluene solution; add 300kg of glycine, 750kg of methanol, control the temperature at 25°C for 2h, control the temperature below 55°C and the pressure at -0.01Mpa to carry out vacuum distillation, and then at 55°C and -0.01MPa Bake for 3 hours to obtain imidic acid; put it into the hydroformylation kettle, add 660kg of phosphorus oxychloride, 250kg of toluene, add 375kg of N,N-dimethyl...
Embodiment 3
[0055] Put 500kg of valeronitrile and 280kg of methanol into the esterification tank, then lower the temperature to 2°C, and feed 280kg of hydrogen chloride gas (prepared by mixing phosphorus trichloride and hydrochloric acid solution according to the mass ratio of 2.0:1.65, and control the pressure in the tank to be lower than 0.1MPa, The temperature is 7-9°C for reaction), after the aeration, keep the temperature at 12°C for 15h, add 250L of sodium hydroxide solution with a mass fraction of 30%, control the temperature below 10°C for 8h to obtain imidate, add 250kg of toluene, Extract to obtain imidate toluene solution; add 260kg of glycine, 750kg of methanol, control the temperature at 25°C for 3h, control the temperature below 55°C and the pressure at -0.01Mpa for vacuum distillation, and then conduct vacuum distillation at 55°C and -0.01MPa Bake for 3 hours to obtain imidic acid; put it into the hydroformylation kettle, add 550kg of phosphorus oxychloride, 220kg of toluene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com