Anti-tumor medicine conjugate, preparation method, preparation and application
A technology of anti-tumor drugs and conjugates, which is applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., to achieve the effect of low preparation cost, high stability, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
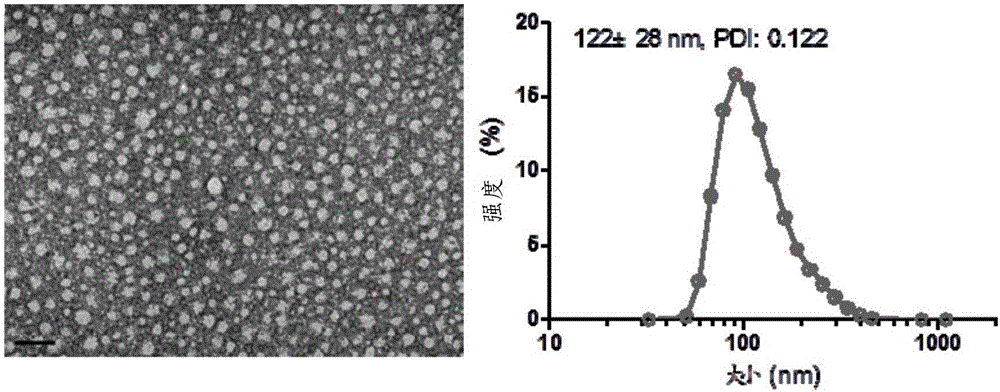
[0061] The synthetic route of SN38-PTX conjugate is as follows figure 1 As shown, the specific steps are as follows:
[0062] PTX (500mg, 0.59mmol) and succinic anhydride (176mg, 1.77mmol) were added to a 100mL round bottom flask, dissolved in 8mL of anhydrous pyridine, then DMAP (7.2mg, 0.06mmol) was added, stirred at 25°C for 3h, and the oil pump Remove pyridine, wash once with 0.1N HCl and saturated saline; dry the organic phase with anhydrous sodium sulfate, filter, collect the filtrate and remove the solvent under reduced pressure; separate and purify the solid by column chromatography (DCM:MeOH=80:1) The product 5 (550 mg, yield 98%) was obtained.
[0063] product 5 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0064] 1 H NMR (400MHz, CDCl 3 ): δ1.14(s,3H),1.23(s,3H),1.69(s,3H),1.89-1.92(d,4H,J=11.2),2.20-2.22(d,4H,J=9.6) ,2.44(s,3H),2.54-2.64(m,4H),2.67-2.70(t,2H),3.68(s,2H),3.80-3.82(d,2H,J=6.8),4.20-4.23( d,1H,J=8.8),4.30-4.32(d,1H,...
Embodiment 2
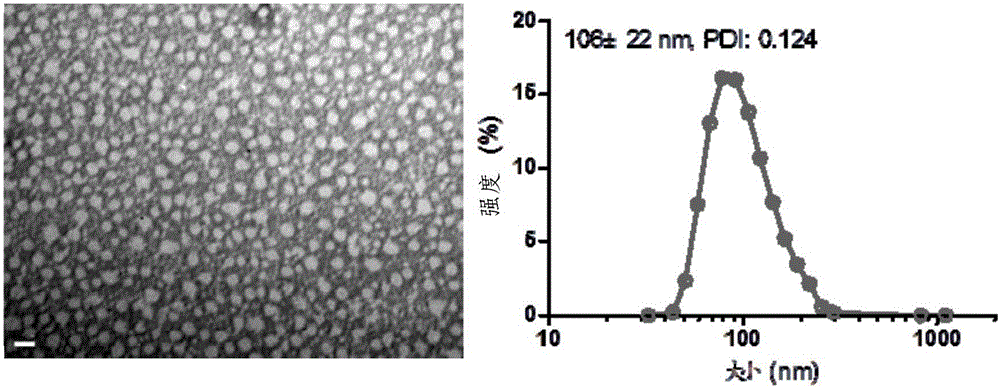
[0071] The synthetic route of SN38-DTX conjugate is as follows figure 2 As shown, the specific steps are as follows:
[0072] Add DTX (500mg, 0.62mmol) and succinic anhydride (186mg, 1.86mmol) into a 100mL round-bottomed flask, dissolve in 8mL of anhydrous pyridine, then add DMAP (7.2mg, 0.06mmol), stir at 25°C for 3h, oil pump Remove pyridine, wash once with 0.1N HCl and saturated saline; dry the organic phase with anhydrous sodium sulfate, filter, collect the filtrate and remove the solvent under reduced pressure; separate and purify the solid by column chromatography (DCM:MeOH=80:1) The product 6 (500 mg, yield 91%) was obtained.
[0073] product 5 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0074] 1 H NMR (400MHz, CDCl 3 ):δ1.08-1.13(t,3H),1.20(s,3H),1.32-1.34(d,9H,J=7.2),1.74-1.76(d,3H,J=6.4),1.83-1.86( d,2H,J=10.0),1.89-1.91(d,3H,J=7.2),2.27-2.28(t,2H),2.37-2.39(d,2H,J=8.4)2.53-2.64(m,5H ),2.77-2.83(m,2H),3.89-3.92(t,1H),4.17-4.20(t...
Embodiment 3
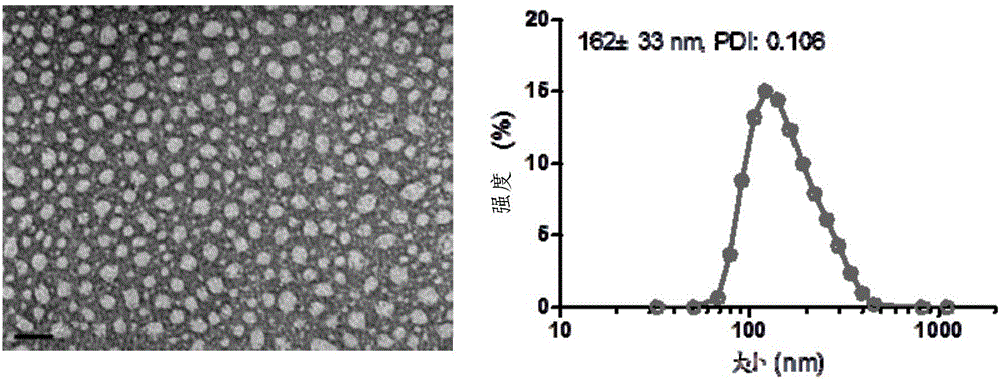
[0081] The synthetic route of SN38-CTX conjugate is as follows image 3 As shown, the specific steps are as follows:
[0082] Add CTX (500mg, 0.60mmol) and succinic anhydride (180mg, 1.80mmol) into a 100mL round-bottom flask, dissolve in 8mL of anhydrous pyridine, then add DMAP (7.2mg, 0.06mmol), stir at 25°C for 3h, oil pump Remove pyridine, add DCM to dissolve, and wash with 0.1N HCl and saturated brine successively; the organic phase is dried with anhydrous sodium sulfate, filtered, and the solvent is removed under reduced pressure after collecting the filtrate; the solid is separated and purified by column chromatography (DCM :MeOH=80:1) to obtain product 7 (549 mg, yield 98%).
[0083] product 7 1 H NMR nuclear magnetic data and mass spectrometry data are as follows:
[0084] 1 H NMR (400MHz, CDCl 3 ): δ1.21-1.22(s,6H),1.36(s,9H),1.57(s,7H),1.72(s,3H),1.87-1.88(d,3H,J=1.2),2.25-2.27 (d,1H,J=9.2),2.66-2.73(m,5H),3.30(s,3H),3.45(s,4H),3.80-3.88(m,2H),4.16-4.18(d,1H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com