Method for preparing water-soluble taxane medicament and application thereof
A taxane, water-soluble technology, applied in the field of water-soluble taxane drugs, can solve problems such as drug dissociation, achieve the effects of reducing toxicity, avoiding toxic side effects and irritation, and improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
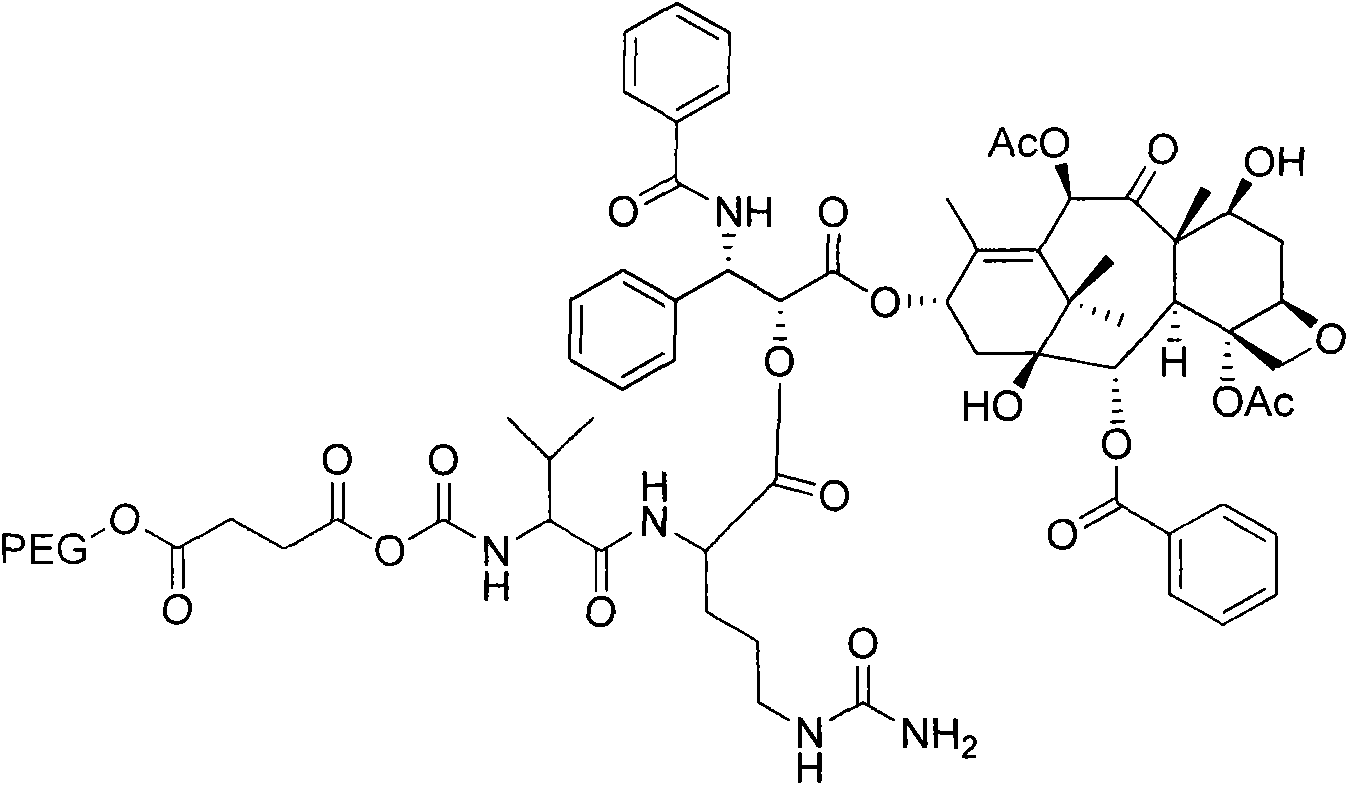
[0062] Embodiment 1: Preparation of PEG-VC-PTX
[0063] Preparation of PEG-succinic acid:
[0064] PEG (20gl), succinic anhydride (0.40g) and triethylamine (1mL) were added to CH 2 Cl 2 (100mL), reflux for 2h, add 100mL of distilled water, with CH 2 Cl 2 Extract (50mL×2), combine CH 2 Cl 2 layer, dried over anhydrous sodium sulfate, filtered, concentrated, added a large amount of diethyl ether to precipitate a solid, filtered with suction, and dried to obtain the product as a white solid, 15.10 g, with a yield of 74.02%.
[0065] Preparation of Fmoc-Val-Cit:
[0066] Dissolve Fmoc-Val (5.06g) and HOSu (1.72g) in THF (100mL), add DCC (3.08g), react for 1h, add Cit (2.74g) and NaHCO 3 (1.32g), reaction 4h.. Add aqueous citric acid solution (30%, 75mL) to the reaction system, extract with ethyl acetate (50mL×2), combine the organic phases, wash with water (50mL×2), evaporate the solvent under reduced pressure, and add Diethyl ether (80 mL) precipitated the product, sucti...
Embodiment 2
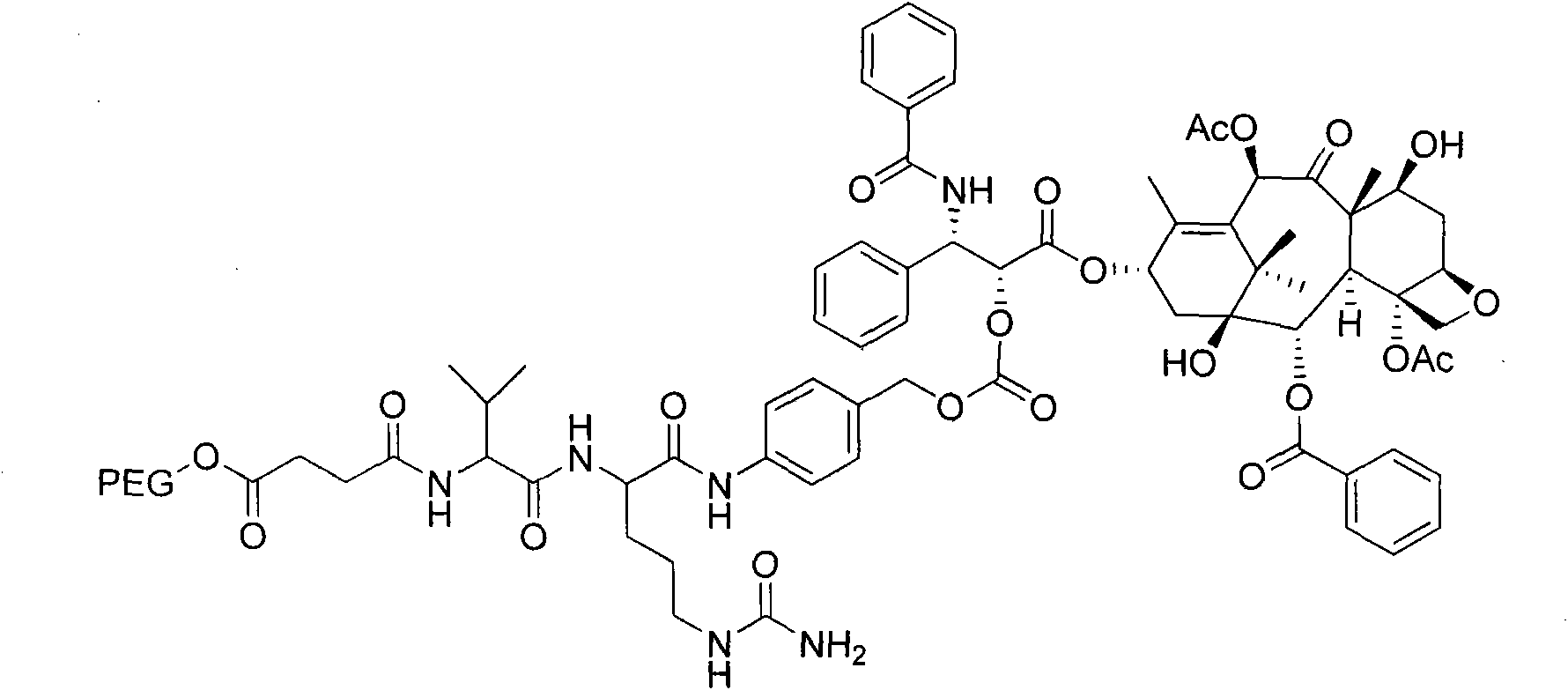
[0071] Embodiment 2: Preparation of PEG-VC-PABC-VC-PTX
[0072] Preparation of Fmoc-Val-Cit-PABOH:
[0073] Add Fmoc-Val-Cit (1.04g), EEDQ (0.52g) and PABOH (0.26mg) to CH 2 Cl 2 , reacted for 10 h, evaporated the solvent under reduced pressure, added diethyl ether, stirred, filtered with suction, washed with diethyl ether, and dried to obtain a light purple solid, 1.10 g, with a yield of 81.84%.
[0074] Preparation of Fmoc-Val-Cit-PABC-PNP:
[0075] Fmoc-Val-Cit-PABOH (0.30g) and PNPCOCl (0.10gl) were added to THF (15mL), triethylamine (300uL) was added dropwise with stirring, and reacted overnight at room temperature. Flash column separation (CHCl 3 :CH 3 OH=20:1→10:1) to obtain a pink foamy solid, 0.20 g, yield 52.31%.
[0076] Preparation of Fmoc-Val-Cit-PABC-PTX:
[0077] Add Fmoc-Val-Cit-PABC-PNP (160 mg), PTX (214 mg) and DMAP (31 mg, 0.25 mmol) sequentially to CH 2 Cl 2 (20mL), react at room temperature for 100h. Flash column (CHCl 3 :CH 3 OH=30:1→20:1) is...
Embodiment 3
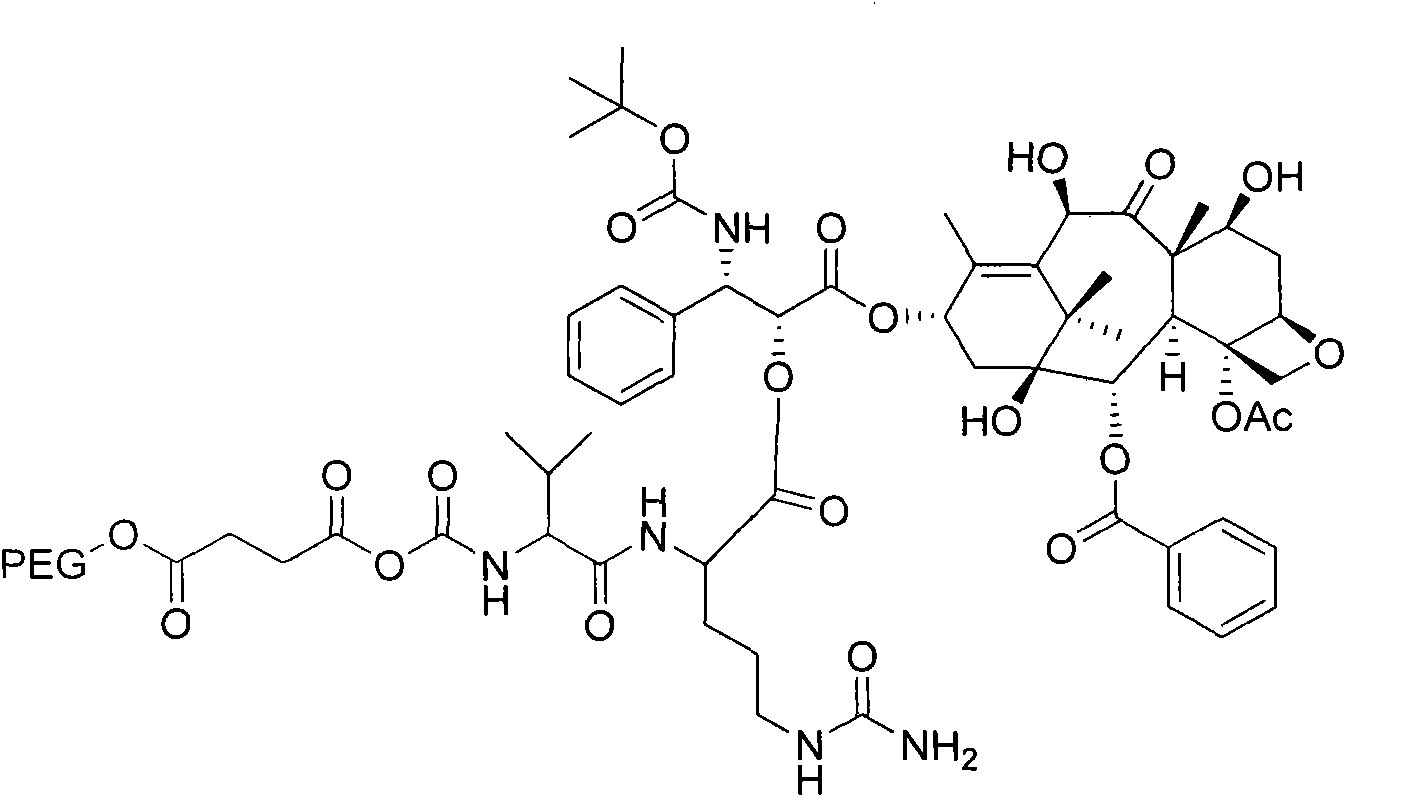
[0080] Embodiment 3: Preparation of PEG-VC-DTX
[0081] Preparation of PEG-succinic acid:
[0082] PEG (20gl), succinic anhydride (0.40g) and triethylamine (1mL) were added to CH 2 Cl 2 (100mL), reflux for 2h, add 100mL of distilled water, with CH 2 Cl 2 Extract (50mL×2), combine CH 2 Cl 2 layer, dried over anhydrous sodium sulfate, filtered, concentrated, added a large amount of diethyl ether to precipitate a solid, filtered with suction, and dried to obtain the product as a white solid, 15.10 g, with a yield of 74%.
[0083] Preparation of Fmoc-Val-Cit:
[0084] Dissolve Fmoc-Val (5.06g) and HOSu (1.72g) in THF (100mL), add DCC (3.08g), react for 1h, add Cit (2.74g) and NaHCO 3 (1.32g), reaction 4h.. Add aqueous citric acid solution (30%, 75mL) to the reaction system, extract with ethyl acetate (50mL×2), combine the organic phases, wash with water (50mL×2), evaporate the solvent under reduced pressure, and add Diethyl ether (80 mL) precipitated the product, suction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com