Entecavir derivative and preparation method thereof
A technology of entecavir and valentecavir, applied in the directions of active ingredients of heterocyclic compounds, antiviral agents, organic chemistry, etc., can solve the problems of low bioavailability of D-amino acid esters and cannot be used as acyclovir prodrugs, etc., and achieves improved pharmacopeia performance, Easy deprotection, low effective dose effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
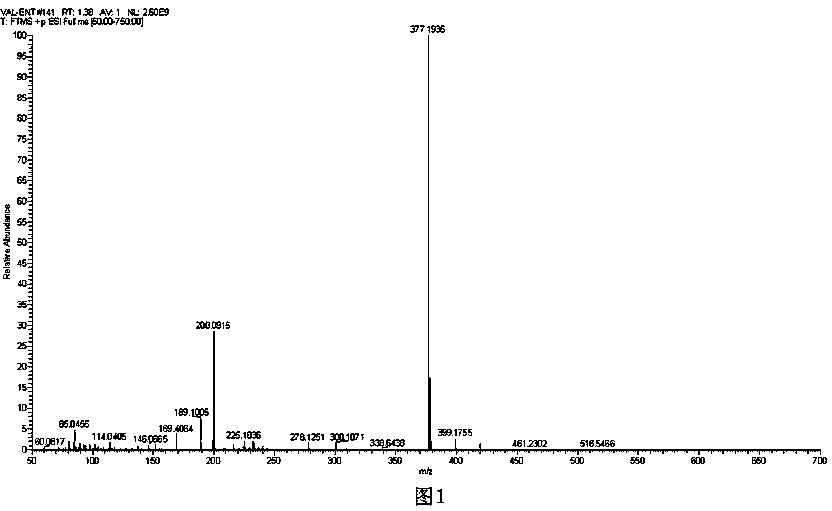
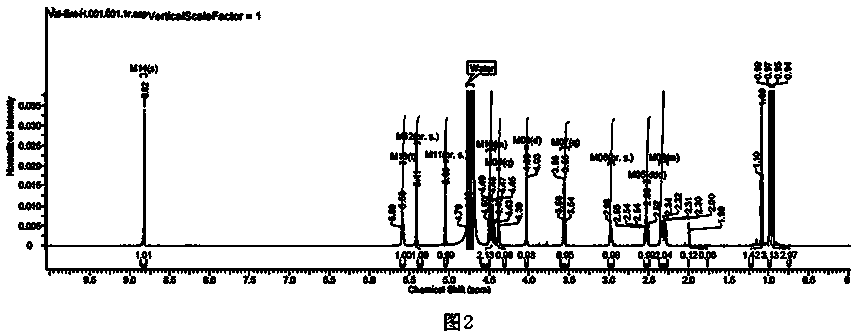
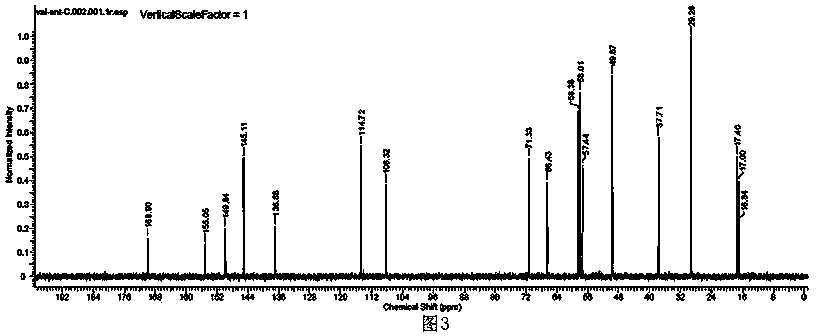
Image
Examples
Embodiment 1
[0052] 1. Preparation of (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid-Entecavir
[0053] Take entecavir: 60.9g, add dimethylformamide 1250mL, shake to dissolve.
[0054] Take (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid: 104.2g, dicyclohexylcarbodiimide: 69.6g, 4-dimethylaminopyridine: 8.2g, add di Methylformamide 1250mL, stirred for 60min, added the above entecavir solution, stirred and reacted at 100°C for 24h.
[0055] After the reaction is completed, filter with suction, add 650 g of silica gel to the filtrate, and dry it on a water bath at 90°C.
[0056] Take 2900g of silica gel, put it on a column, put the fried silica gel on the column, wash it with methanol-ethyl acetate (10:90), and collect every 12L. TLC monitors the fractions, merges the fractions containing the target product, and evaporates to dryness under reduced pressure to obtain (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid-entecavir, i.e. Boc-protected valeric acid Amino ...
Embodiment 2
[0066] 1. Preparation of (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid-Entecavir
[0067] Take entecavir: 60.9g, add dimethylformamide 2500mL, shake to dissolve.
[0068] Take (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid: 52.1g, dicyclohexylcarbodiimide: 34.8g, 4-dimethylaminopyridine: 4.1g, add di Methylformamide 2500mL, stirred for 30min, added the above entecavir solution, stirred and reacted at 100°C for 1h.
[0069] After the reaction is completed, filter with suction, add 650 g of silica gel to the filtrate, and dry it on a water bath at 90°C.
[0070] Take 2900g of silica gel, put it on a column, put the fried silica gel on the column, wash it with methanol-ethyl acetate (10:90), and collect every 12L. The fractions were monitored by TLC, and the fractions containing the target product were combined and evaporated to dryness under reduced pressure to obtain (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid-entecavir.
[0071] , 2-amino-...
Embodiment 3
[0075] 1. Preparation of (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid-Entecavir
[0076] Take entecavir: 60.9g, add dimethylformamide 1250mL, shake to dissolve.
[0077] Take (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid: 208.4g, dicyclohexylcarbodiimide: 139.2g, 4-dimethylaminopyridine: 16.4g, add di Methylformamide 1250mL, stirred for 60min, added the above entecavir solution, stirred and reacted at 100°C for 72h.
[0078] After the reaction is completed, filter with suction, add 650 g of silica gel to the filtrate, and dry it on a water bath at 90°C.
[0079] Take 2900g of silica gel, put it on a column, put the fried silica gel on the column, wash it with methanol-ethyl acetate (10:90), and collect every 12L. The fractions were monitored by TLC, and the fractions containing the target product were combined and evaporated to dryness under reduced pressure to obtain (S)-2-(tert-butoxycarbonyl-amino)-3-methylbutanoic acid-entecavir.
[0080] , 2-am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com