Tumor-targeting pH- and redox-response macromolecule nano prodrug and preparation method and application thereof
A tumor-targeting, dual-response technology, applied in anti-tumor drugs, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve problems such as unrealistic, insufficient cumulative concentration to kill tumor cells, and increased injection dose, etc. achieve high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
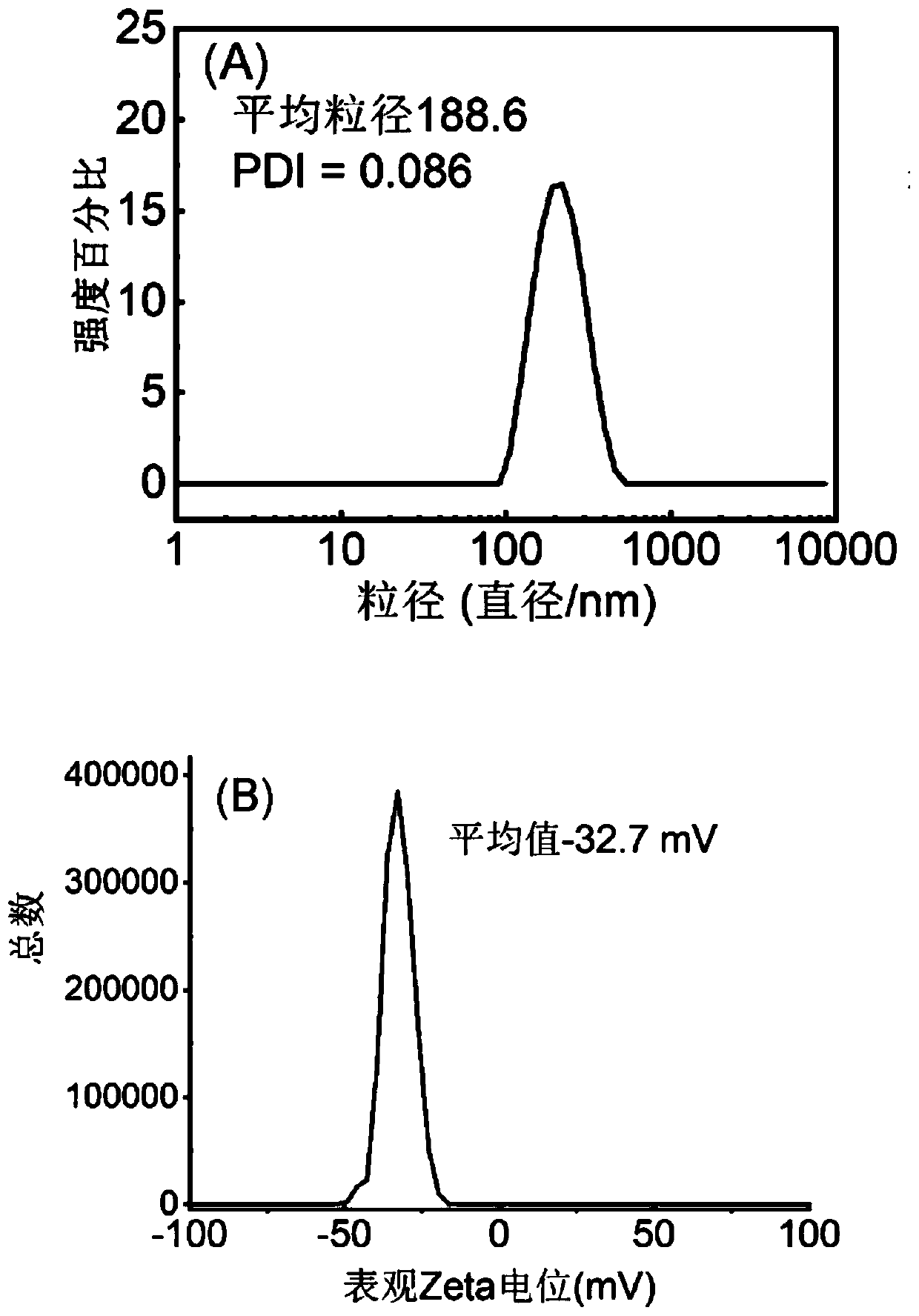
Examples
Embodiment 1
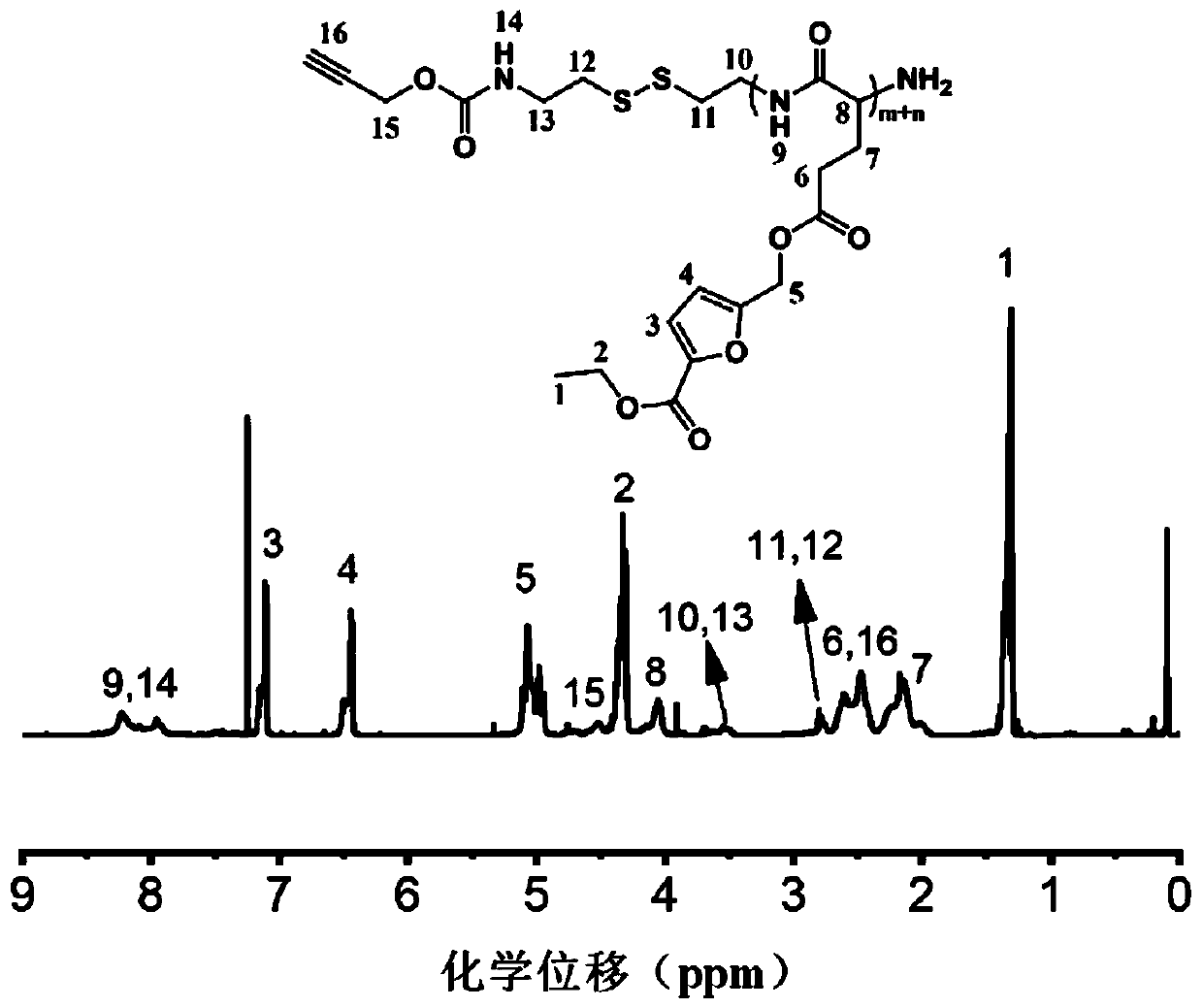
[0077] Embodiment 1: Synthesis of the terminal azide hyaluronic acid in formula (1)
[0078] Dissolve hyaluronic acid (4.8 parts by mass) in 145 parts by volume of acetic acid-sodium acetate buffer solution (pH5.6), then add azidopropylamine (4.9 parts by mass) and (2.8 parts by mass) cyano Sodium borohydride, stirred at room temperature for 7 days. After the mixture was concentrated, it was dialyzed with a dialysis bag (3.5 kDa), acidified with hydrochloric acid, stirred for 30 min, dialyzed again, and a white solid product was obtained after freeze-drying.
Embodiment 2
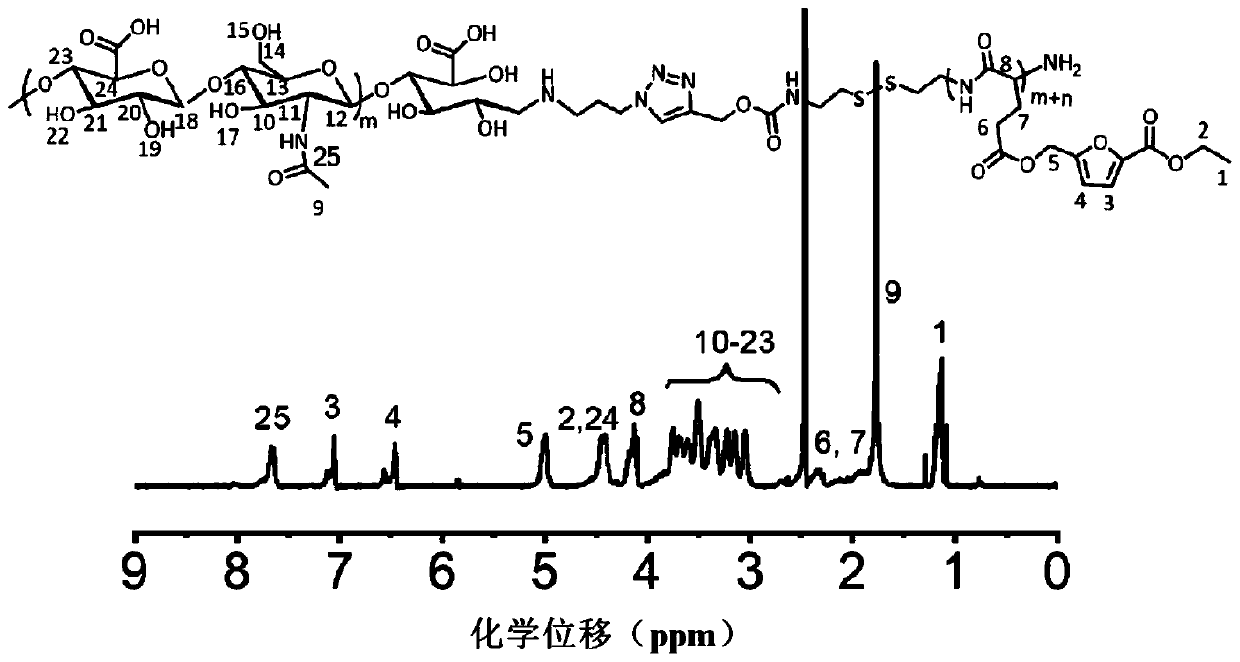
[0079] Embodiment 2: the synthesis of the glutamic acid esterification product containing furan ring in formula (two)
[0080] Copper acetate monohydrate (41.2 parts by mass) and glutamic acid (29.4 parts by mass) were dissolved in 750 parts by volume of water, respectively. Under the condition of 70°C, the copper acetate aqueous solution was added dropwise to the glutamic acid aqueous solution, and reacted at room temperature for 48 hours after the dropwise addition was completed. The precipitate was filtered out with suction and washed with appropriate amount of water and ethanol respectively. Vacuum drying at 70°C for 24 hours yielded chelated copper glutamic acid with a yield of 90%.
[0081] Glutamic acid (5.5 parts by mass) and copper glutamate chelated copper (3.38 parts by mass) were suspended in a mixed solution of DMF (2 parts by volume) and 4 parts by volume of water, and then added dropwise in 1,1,3 , 3-Tetramethylguanidine (5.8 parts by volume). Stirring of the m...
Embodiment 3
[0082] Embodiment 3: the synthesis of acid anhydride monomer in the N-carboxyl ring in formula (three)
[0083] Suspend 0.05 mole parts of the glutamic acid esterification product with dry ethyl acetate. When the temperature rises to 70 °C, add 0.020 mole parts of triphosgene in batches. N 2 bubbling. After the reaction, the reaction solution was cooled to room temperature, and extracted once with ice water, 0.5% sodium bicarbonate and ice water respectively. The organic phase was dried with anhydrous sodium sulfate, and the solvent was rotary evaporated to dryness after filtering off the sodium sulfate. The product was pale yellow flocculent crystals, and the yield was 45%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com