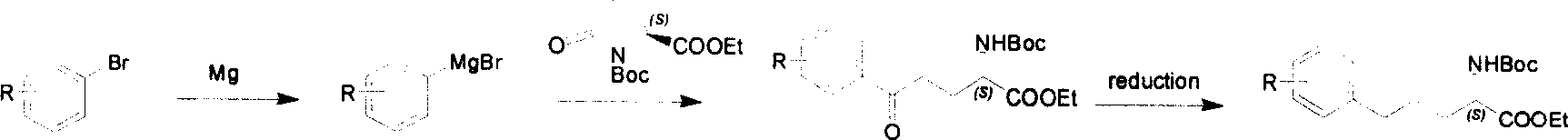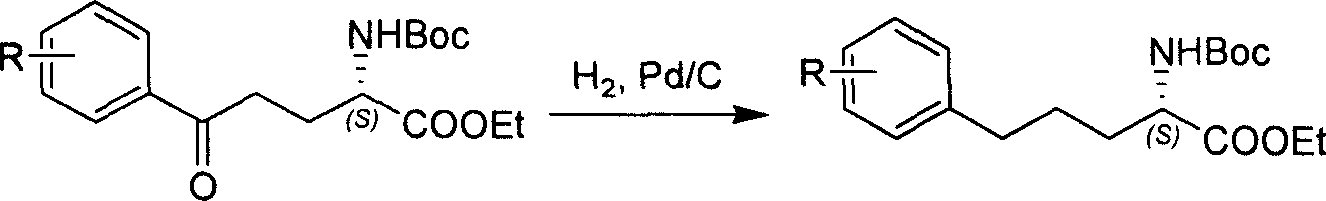Method for synthesizing optically active derivative of 5 - aryl - (S) - N - boc - alpha amino pentanoic acid
A tert-butoxycarbonyl, optically active technology, applied in the field of synthesis of 5-aryl--N-tert-butoxycarbonyl-α-aminovaleric acid derivatives, can solve the problem of reducing synthesis cost, incapable of industrialized production, and synthesis time. Long and other problems, to achieve the effect of shortening the synthesis time and selecting a reasonable reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 【5-Phenyl-5-keto-(S)-N-tert-butoxycarbonyl-α-aminovalerate ethyl ester (3a)】Synthesis:
[0027]
[0028] First prepare the Grignard reagent (2a) of bromobenzene, equipped with a dropping funnel and a three-necked flask with an internal thermometer, put Mg (0.77g, 32mmol) and dried tetrahydrofuran (30mL), and replace it with nitrogen, A solution of bromobenzene (5.1 g, 32 mmol) in dry tetrahydrofuran (10 mL) was prepared in a dropping funnel. Start to drop 2mL of this solution to initiate the reaction, then slowly drop the rest, maintaining the internal temperature at 60-70°C.
[0029] N-tert-butoxycarbonyl-L-pyroglutamic acid ethyl ester (1) (7g, 27mmol) in dry tetrahydrofuran (100mL) was cooled to between minus 50°C and minus 40°C with dry ice, acetone and water system, drop Add the above-mentioned Grignard reagent 2a (40mL, 32mmol), after the addition is completed, the reaction solution is stirred at this temperature for 60 minutes, rises to minus 10°C, and is the...
Embodiment 2
[0034] Synthesis of [5-p-methoxyphenyl-5-one-(S)-N-tert-butoxycarbonyl-α-aminovaleric acid ethyl ester (3b)]:
[0035]
[0036]N-tert-butoxycarbonyl-L-pyroglutamic acid ethyl ester (1) (4.6g, 18mmol) in dry tetrahydrofuran (100mL) was cooled to between minus 50°C and minus 40°C with dry ice, acetone and water, Grignard reagent (2b) (40mL, 20mmol) of p-methoxybromobenzene was added dropwise, and the dropwise addition was completed. The reaction solution was stirred at this temperature for 60 minutes, raised to minus 10°C, and then washed with 10% saturated NH 4 Cl aqueous solution was quenched, the organic layer was separated, the aqueous layer was extracted three times with 50 mL of diethyl ether, the combined organic layer was washed with water and saturated brine, and then washed with anhydrous Na 2 SO 4 Drying, filtration, rotary evaporation, column chromatography (petroleum ether / ethyl acetate=20:1) to obtain the target product 5-p-methoxyphenyl-5-one-(S)-N-tert-butoxy...
Embodiment 3
[0041] Synthesis of [5-p-methoxyphenyl-5-one-(S)-N-tert-butoxycarbonyl-α-aminovaleric acid ethyl ester (3b)]:
[0042]
[0043] N-tert-butoxycarbonyl-L-pyroglutamic acid ethyl ester (1) (4.6g, 18mmol) in dry tetrahydrofuran (100mL) was cooled to between minus 50°C and minus 40°C with dry ice, acetone and water, Grignard reagent (2b) (40mL, 20mmol) of p-methoxybromobenzene was added dropwise, and the dropwise addition was completed. The reaction solution was stirred at this temperature for 60 minutes, raised to minus 10°C, and then washed with 10% saturated NH 4 Cl aqueous solution was quenched, the organic layer was separated, the aqueous layer was extracted three times with 50 mL of diethyl ether, the combined organic layer was washed with water and saturated brine, and then washed with anhydrous Na 2 SO 4 Drying, filtration, rotary evaporation, column chromatography (petroleum ether / ethyl acetate=20:1) to obtain the target product 5-p-methoxyphenyl-5-one-(S)-N-tert-butox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com