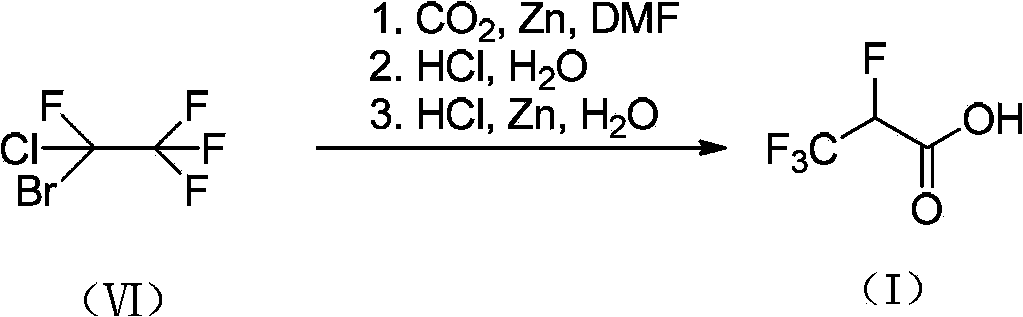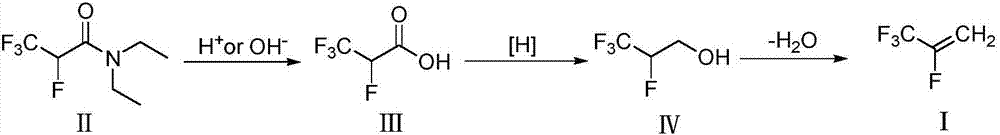Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
167results about "Preparation from carboxylic acid amides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
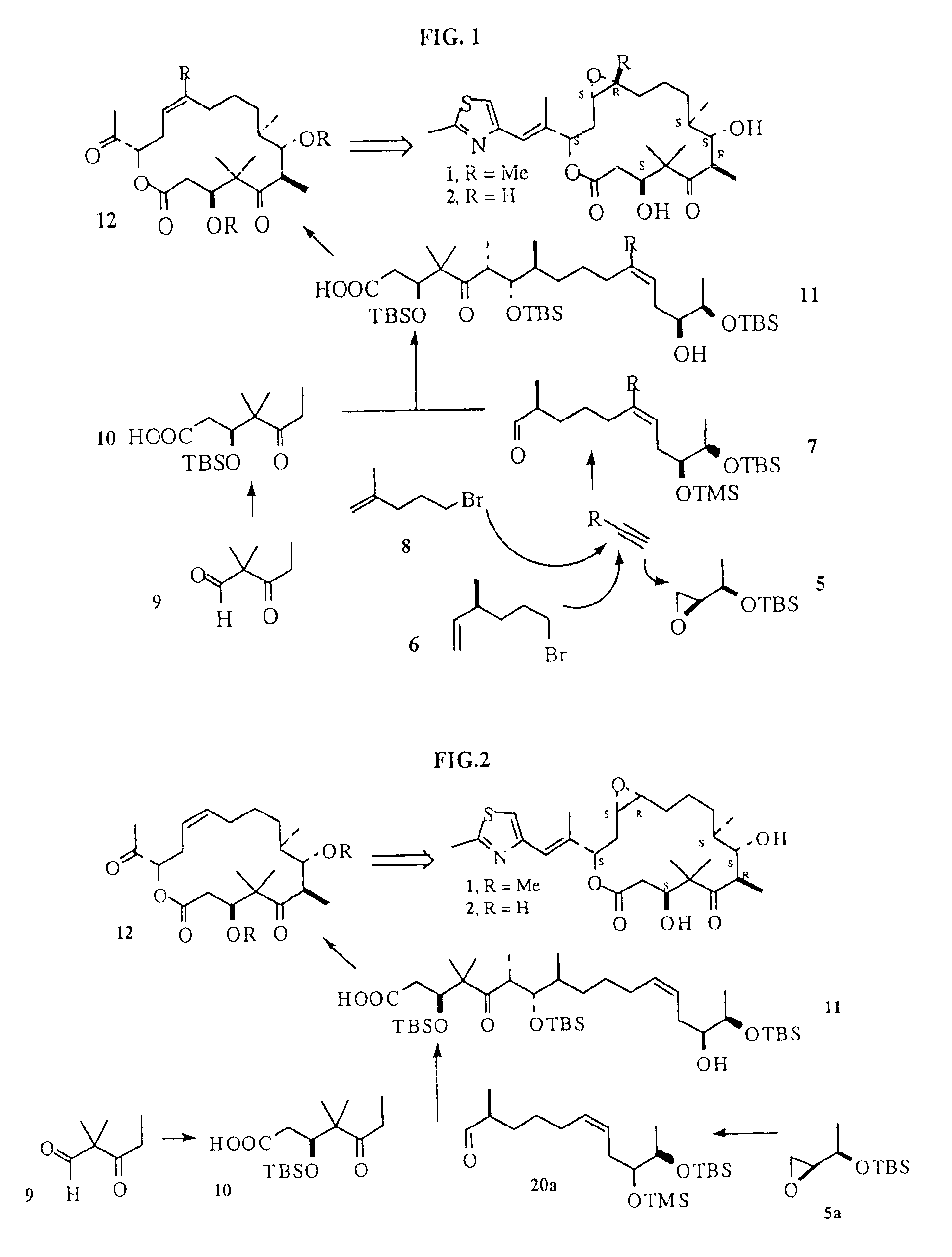
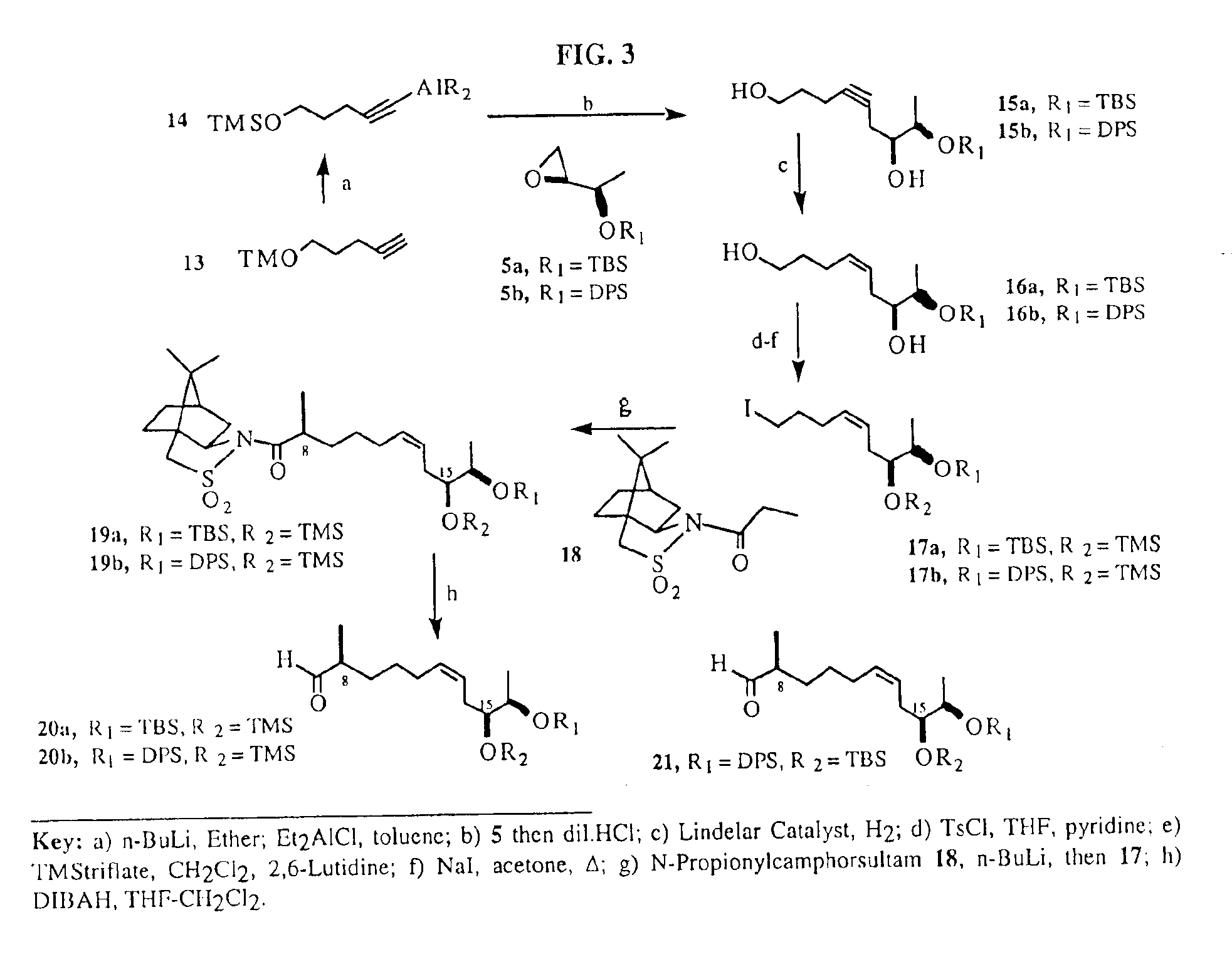
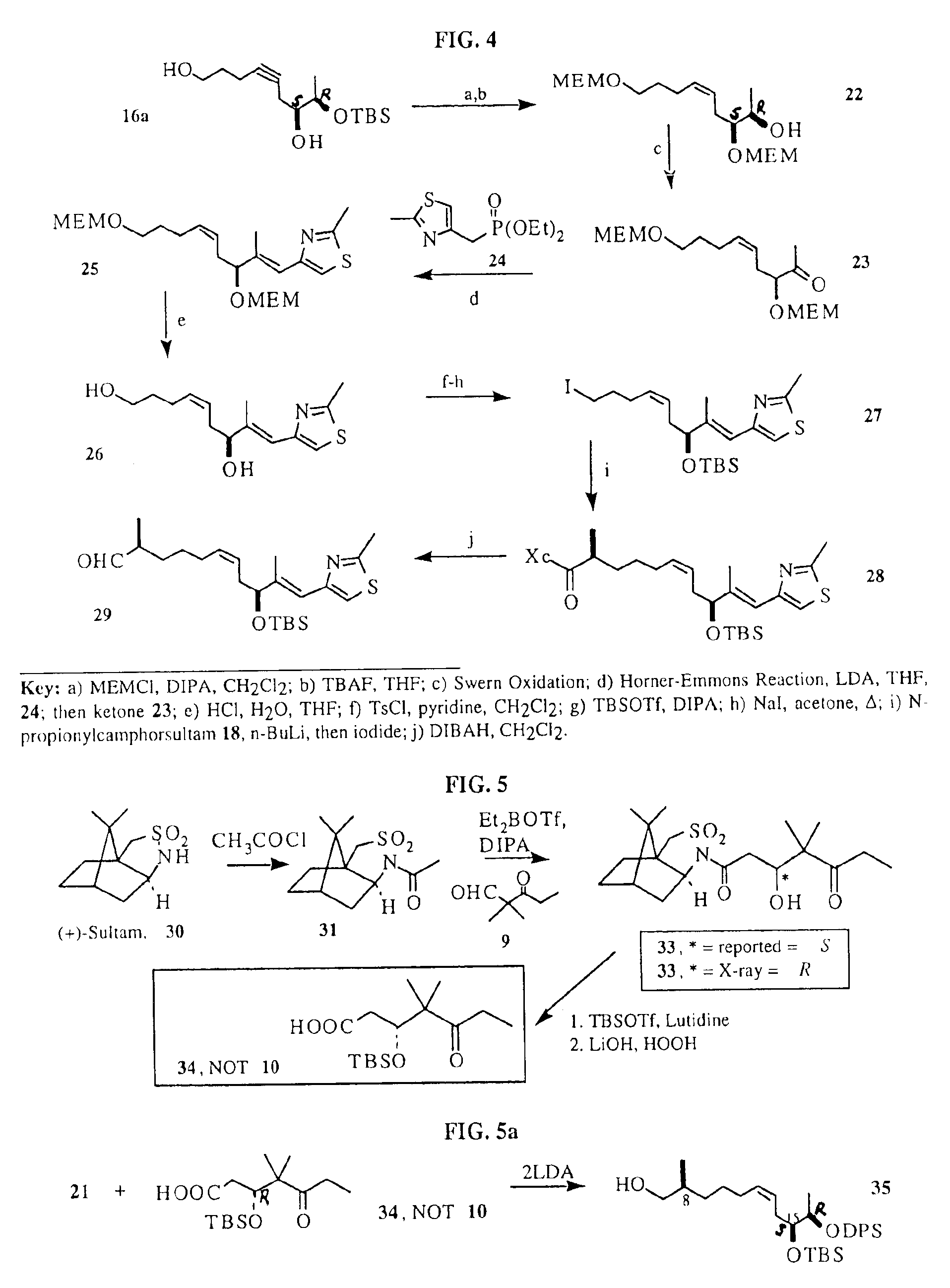
Synthesis of epothilones and related analogs
InactiveUS6989450B2Easy to understandOrganic active ingredientsGroup 4/14 element organic compoundsEpothiloneStereoisomerism
The present invention relates to methods for use in producing epothilones and analogs and derivatives thereof. A general method according to the present invention broadly comprises performing an aldol condensation of a first compound with a second compound thereby to form a third compound selected from the formulas: and stereoisomers thereof, and performing a macrolactonization of the third compound. The present invention also provides chemical compounds, and methods for producing such chemical compounds, that are useful in producing epothilones and analogs and derivatives thereof.
Owner:UNIVERSITY OF MISSISSIPPI
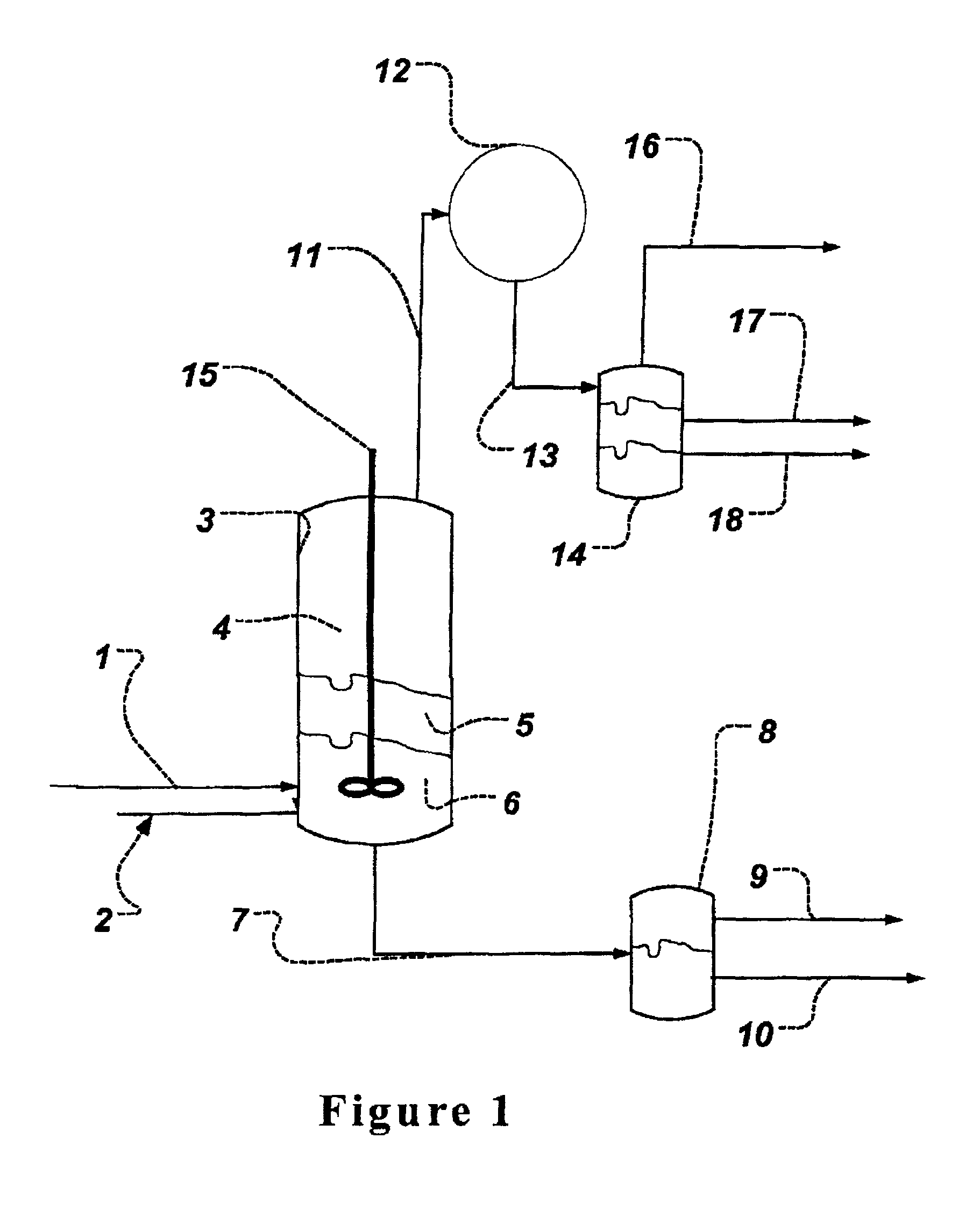
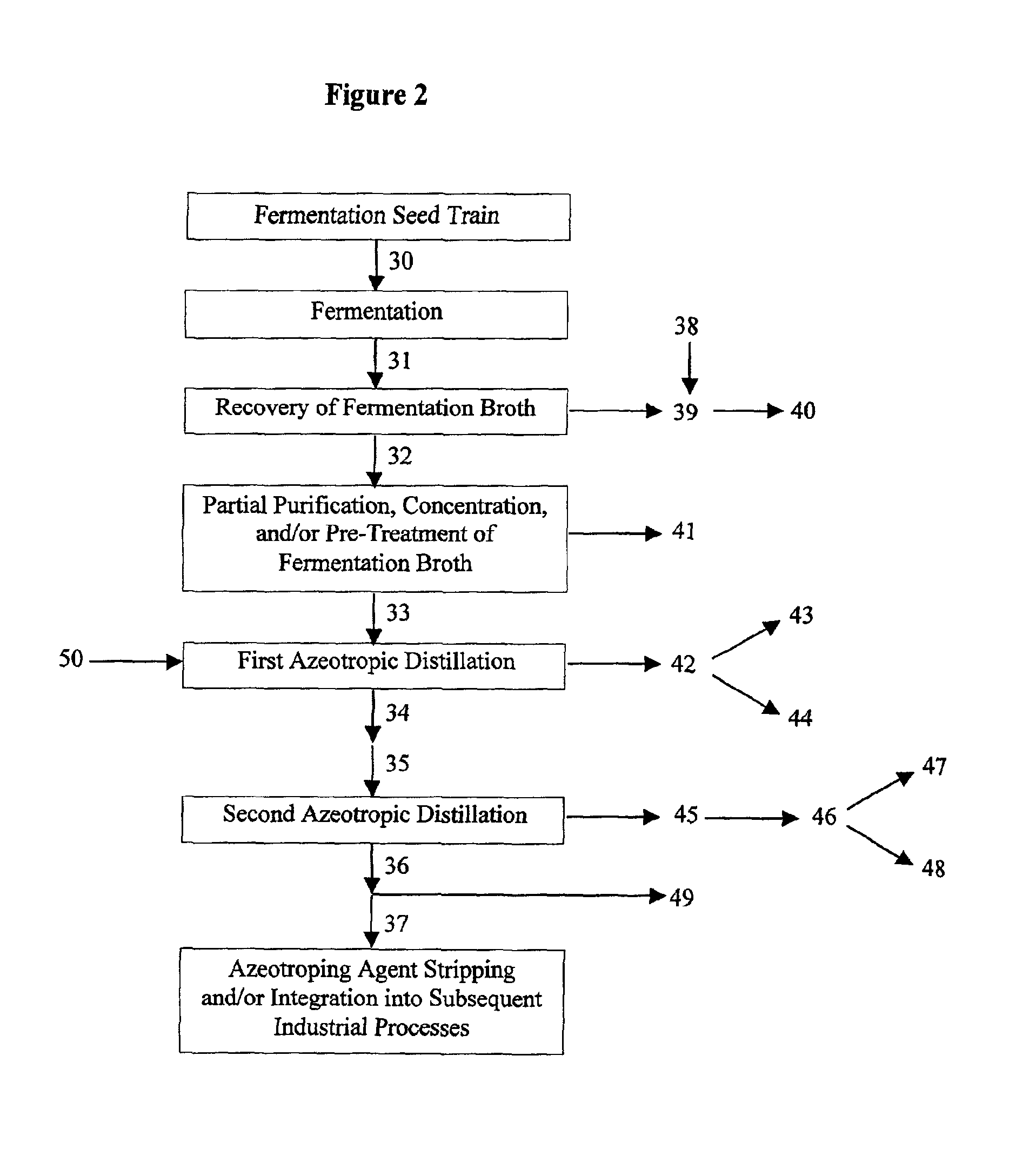
Process for obtaining an organic acid from an organic acid ammonium salt, an organic acid amide, or an alkylamine organic acid complex
InactiveUS6926810B2Simple and cost-effective recoveryPreparation from carboxylic acid saltsOrganic compound preparationOrganic acidHeteroazeotrope
Disclosed herein are methods for the recovery of an organic acid, such as a heat stable lactic acid, from a feed stream which contains at least one of an organic acid amide, an organic acid ammonium salt, or an alkylamine-organic acid complex. The feed stream is mixed with at least one azeotroping agent. The azeotroping agent is a hydrocarbon capable of forming at least one azeotrope with the organic acid that is produced by the thermal decomposition of the amide, ammonium salt, or complex in the feed stream. Preferably the azeotrope is a heteroazeotrope. The mixture of the feed stream and the azeotroping agent is heated to produce a vapor stream. The azeotrope is a component of the vapor stream. The vapor stream can be condensed to a liquid stream, and the organic acid is recovered in the liquid stream that is produced. When the azeotrope is a heteroazeotrope, the vapor stream can be condensed into a liquid stream, which can be separated into a first phase and a second phase. The first phase contains the highest concentration of organic acid and the second phase contains azeotroping agent. The organic acid can be further purified and / or concentrated from the separated first phase or from the liquid stream.
Owner:TATE & LYLE INGREDIENTS AMERICAS INC
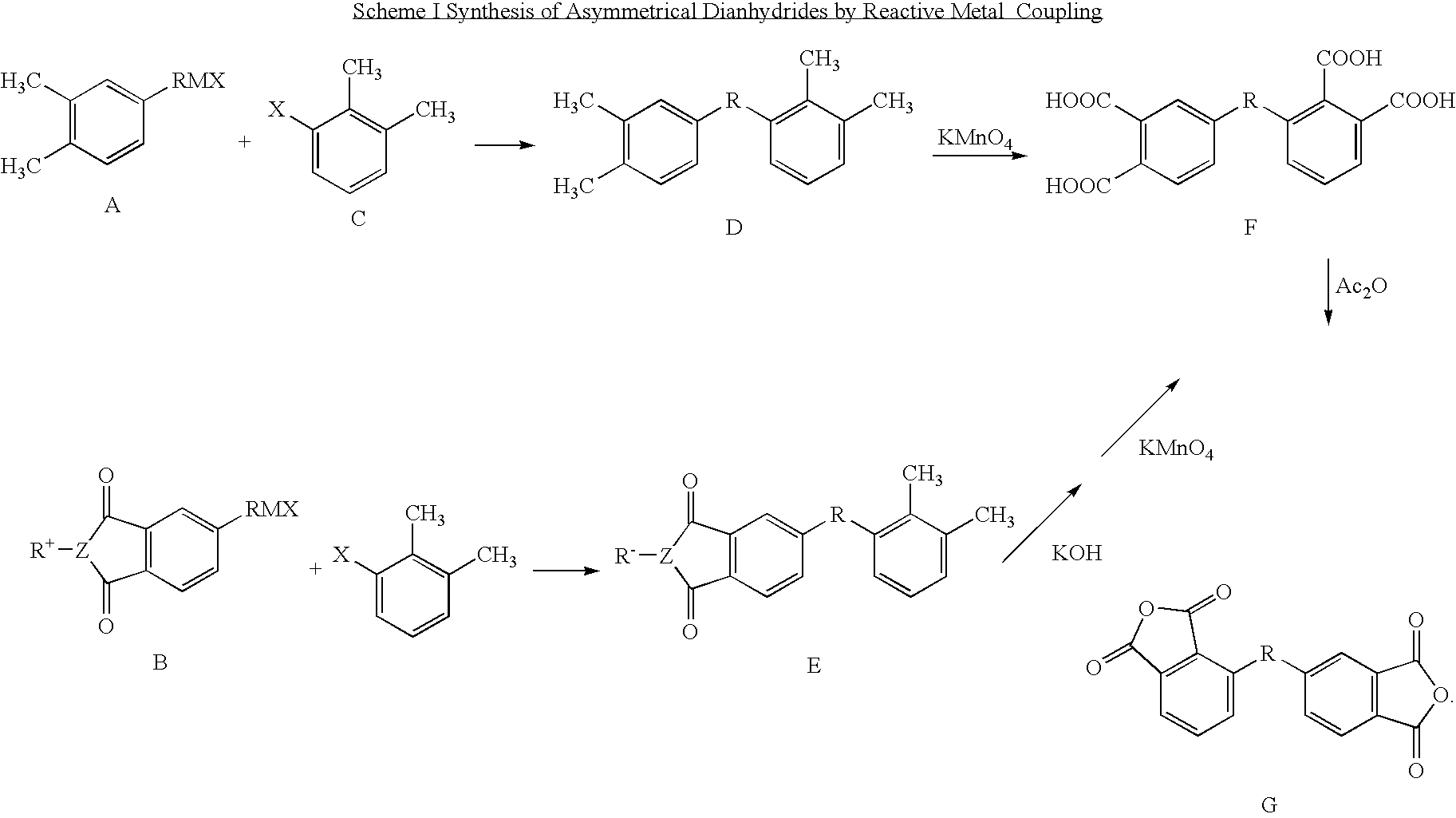
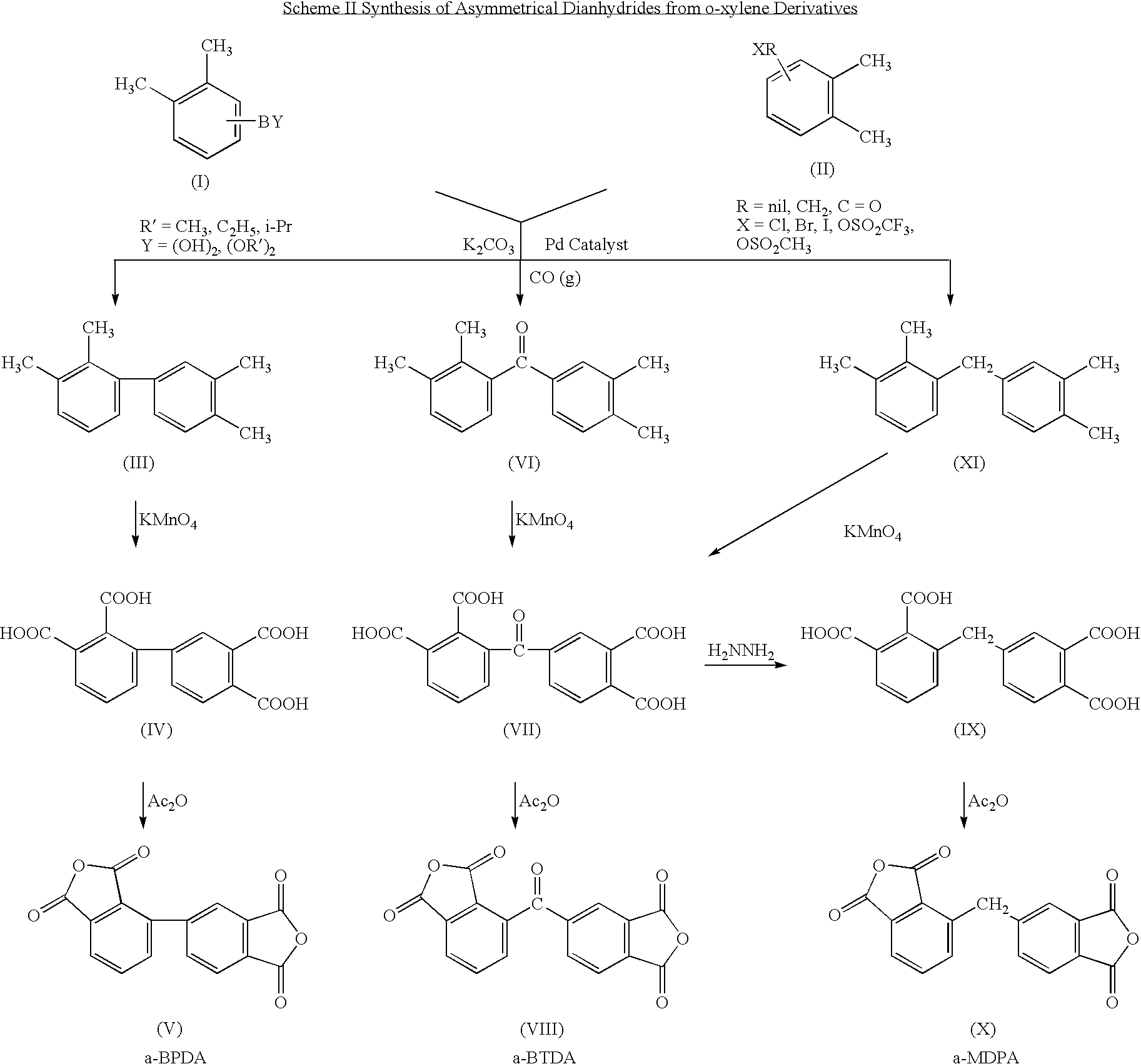
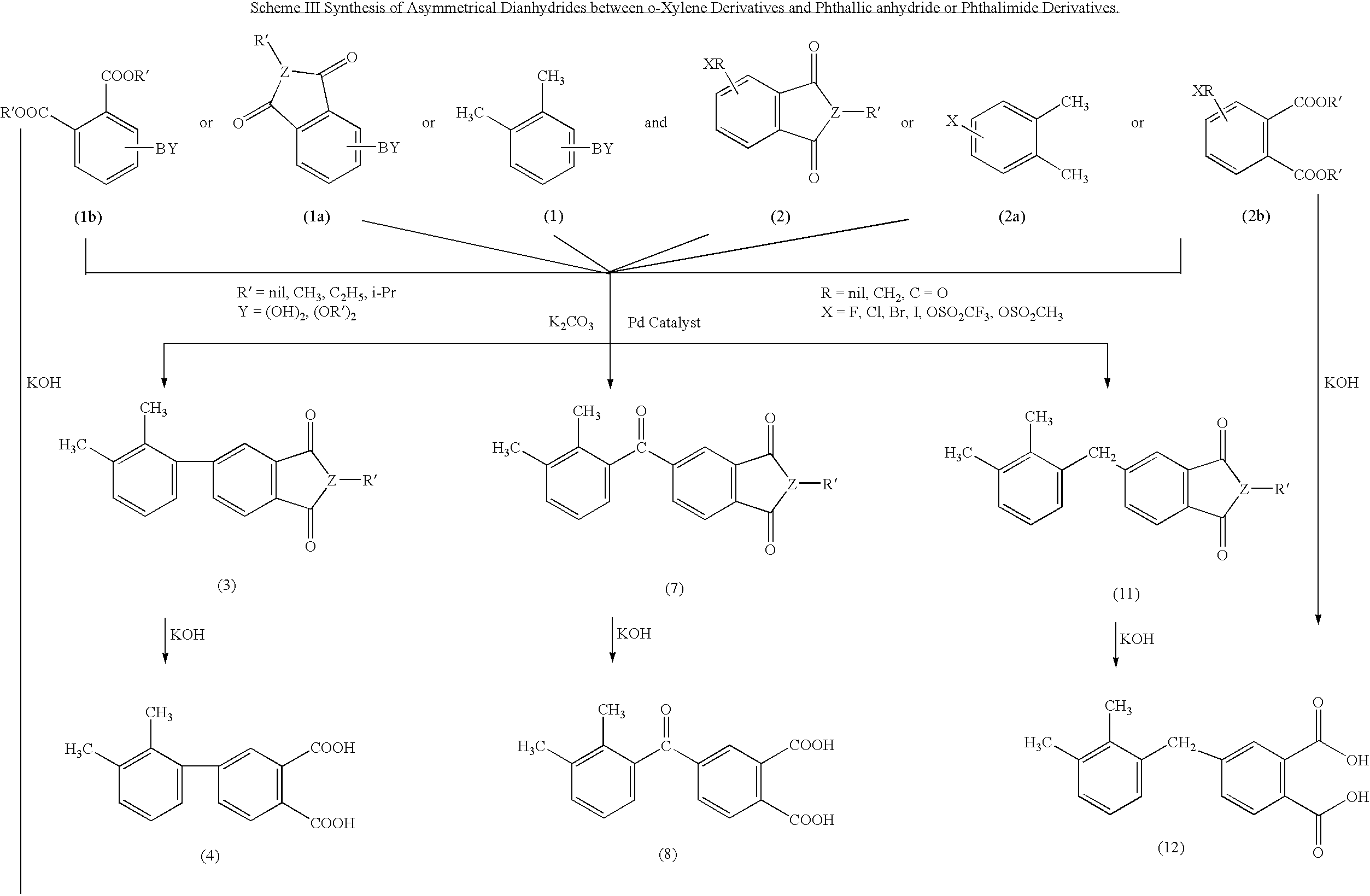
Synthesis of asymmetric tetracarboxylic acids and corresponding dianhydrides
ActiveUS7425650B1High yieldOrganic compound preparationPreparation from carboxylic acid amidesO-XyleneBPDA
This invention relates to processes for preparing asymmetrical biphenyl tetracarboxylic acids and the corresponding asymmetrical dianhydrides, namely 2,3,3′,4′-biphenyl dianhydride (a-BPDA), 2,3,3′,4′-benzophenone dianhydride (a-BTDA) and 3,4′-methylenediphthalic anhydride (-MDPA). By cross-coupling reactions of reactive metal substituted o-xylenes or by cross-coupling o-xylene derivatives in the presence of catalysts, this invention specifically produces asymmetrical biphenyl intermediates that are subsequently oxidized or hydrolyzed and oxidized to provide asymmetric biphenyl tetracarboxylic acids in comparatively high yields. These asymmetrical biphenyl tetracarboxylic acids are subsequently converted to the corresponding asymmetrical dianhydrides without contamination by symmetrical biphenyl dianhydrides.
Owner:UNITED STATES GOVERNMENT ADMINSRATOR OF NAT AERONAUTICS & SPACE
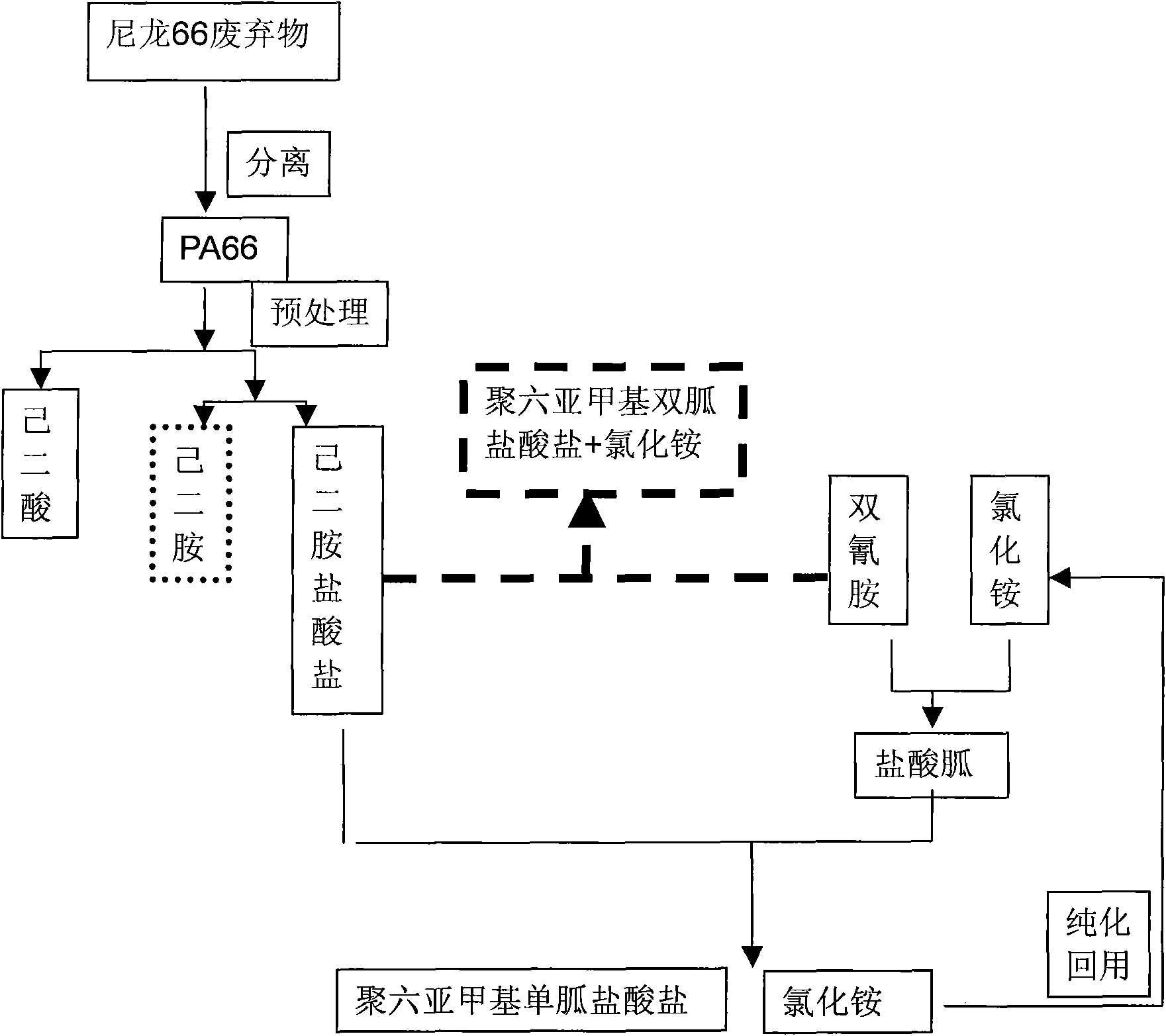
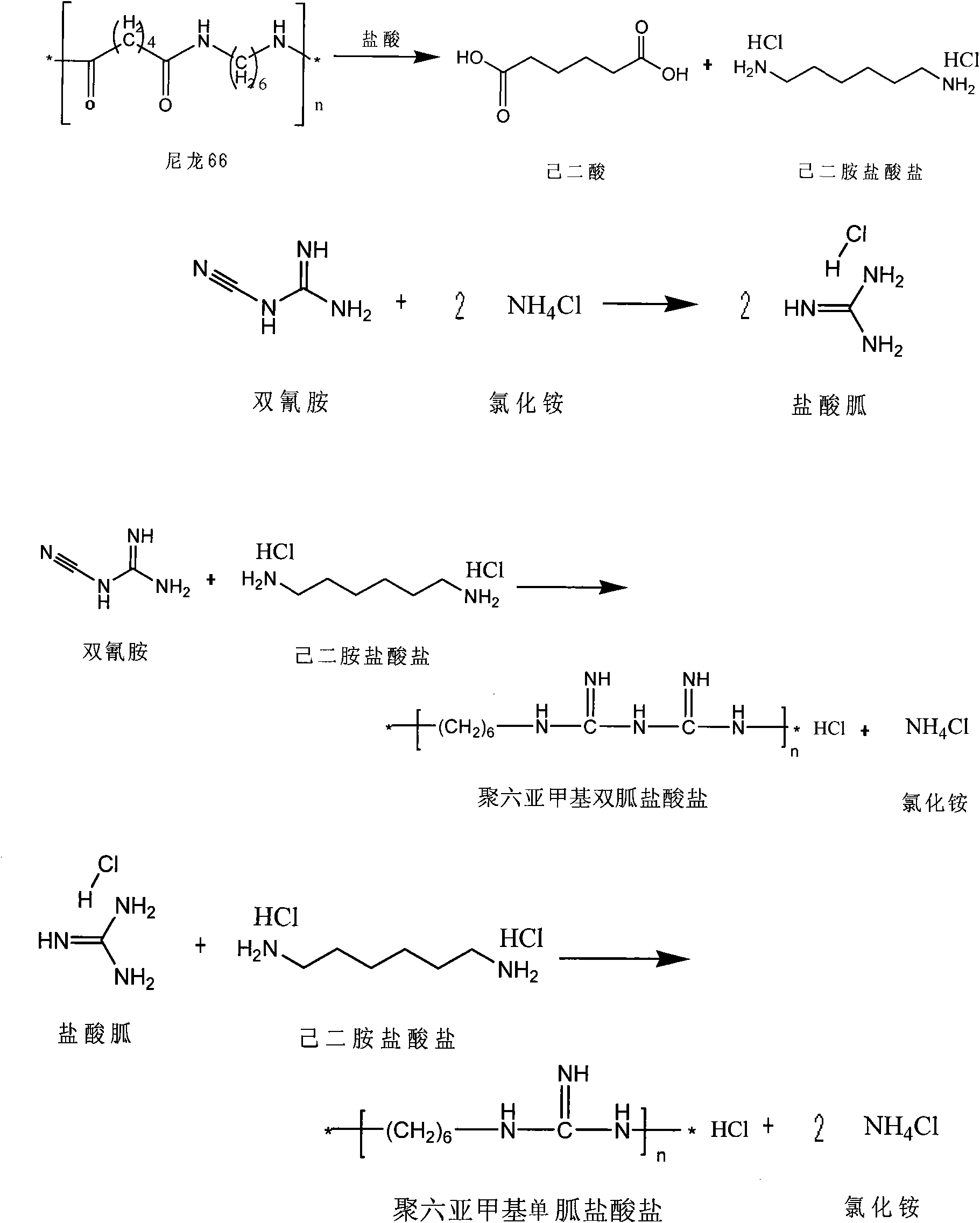
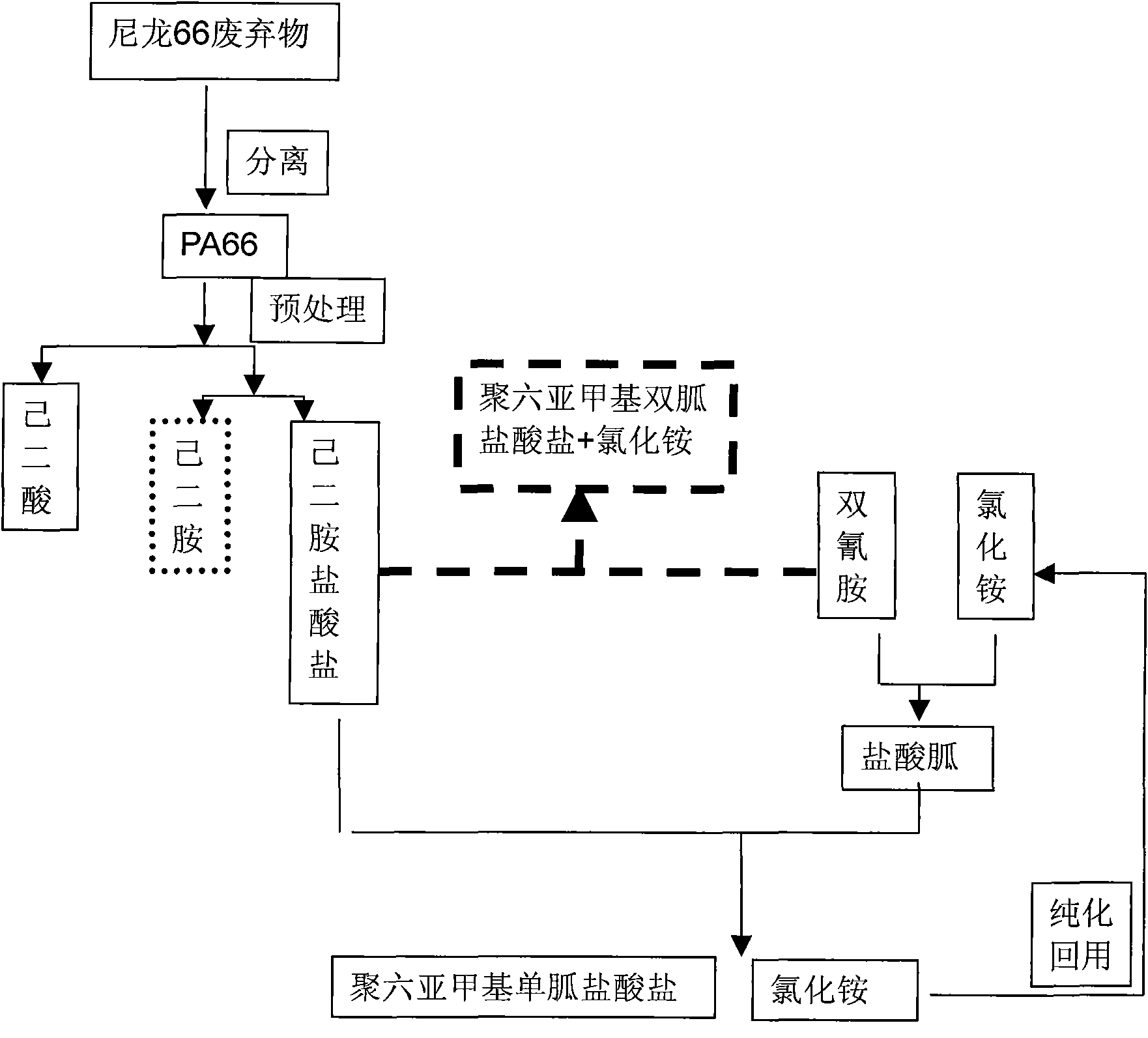
Method for producing adipic acid, hexamethylenediamine hydrochloride and polyhexamethylene (di)guanidine chloride from nylon-66 through depolymerization
InactiveCN101857540ASolving RecyclingSolve the problem of the sourceBiocideOrganic active ingredientsDepolymerizationHexamethylenediamine
The invention discloses a method for producing adipic acid, hexamethylenediamine hydrochloride and polyhexamethylene (di)guanidine chloride from waste nylon-66 serving as a raw amerial through depolymerization, which is characterized by comprising the following steps of: depolymerizing the waste nylon-66 by using hydrochloric acid to obtain refined adipic acid and hexamethylenediamine hydrochloride; polymerizing the hexamethylenediamine hydrochloride and dicyandiamide (in a molar ratio of 1:1) at the temperature of between 170 and 190DEG C for 1 to 3 hours to obtain a white or pale yellow solid polyhexamethylene diguanidine chloride; and adding dicyandiamide and ammonium chloride powder in a molar ratio of 1:2 into a reactor, performing reaction at high temperature for 1 to 3 hours, slowly adding hexamethylenediamine hydrochloride powder, continuously reacting for 3 to 7 hours to obtain a white or pale yellow solid polyhexamethylene guanidine chloride. The method improves production efficiency, reduces production cost, and solves the problems of difficult separation of the product hexamethylenediamine due to depolymerization, low yield and poor benefit; and the technological process is environment-friendly, simple and convenient, and is suitable for industrial production.
Owner:NINGBO UNIV
Method of and apparatus for decomposing wastes
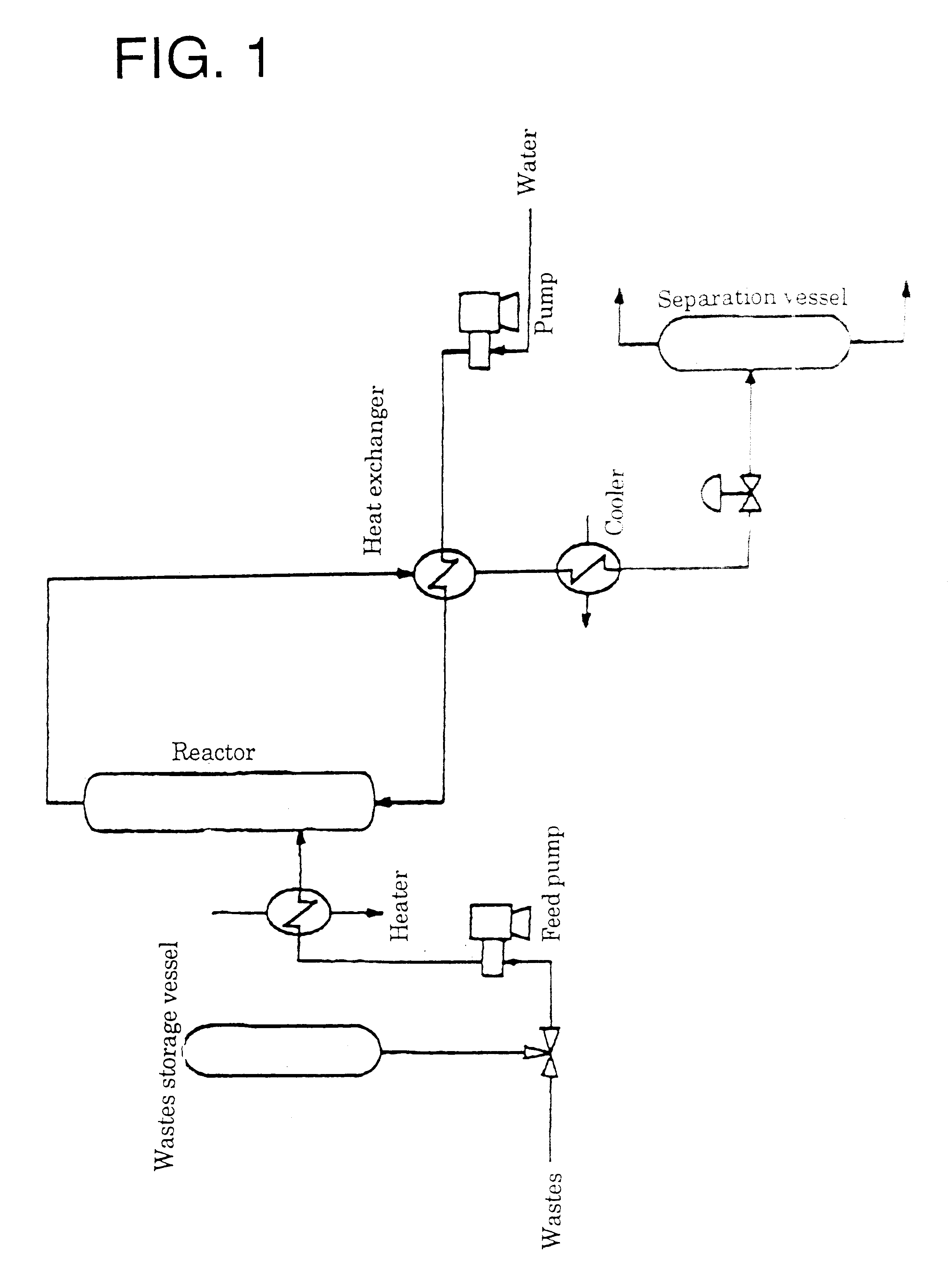
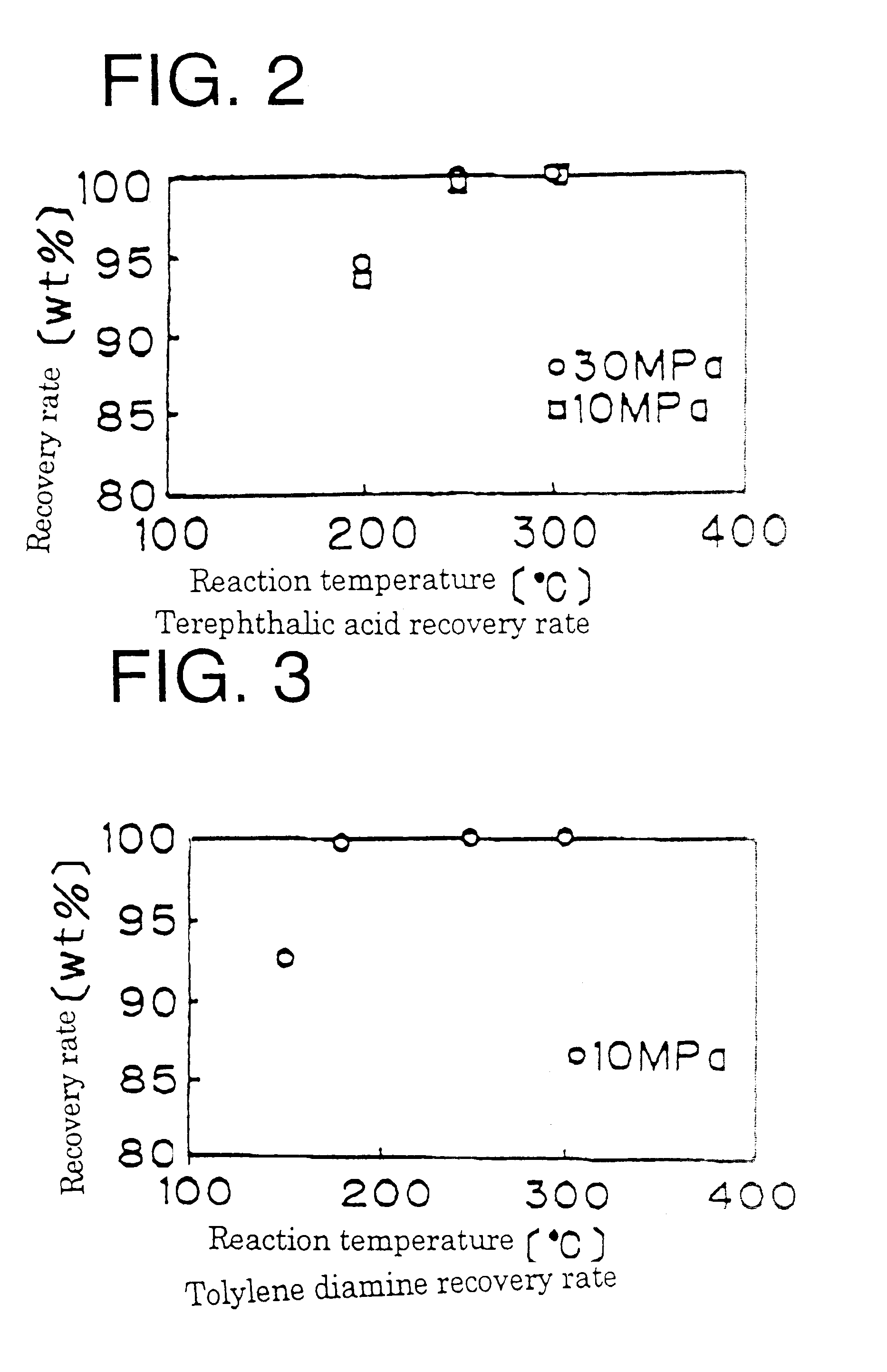
InactiveUS6462230B1Useful operationResultant decomposition products can be utilized effectivelyOrganic compound preparationPreparation from carboxylic acid esters/lactonesMolten stateLiquid state
A method of decomposing wastes containing target compounds having one or more of hydrolyzable bonds of ether bond, ester bond, amide bond and isocyanate bond wherein the method comprises continuously supplying the wastes in a molten state or liquid state to a reactor, continuously supplying super-critical water or high pressure / high temperature water to the reactor, bringing the water into contact with the wastes, thereby decomposing the target compounds and then recovering them as raw material compounds or derivatives thereof for the target compounds. Target compounds contained in wastes in chemical plants which could not be utilized but merely incinerated or discarded so far are continuously decomposed into raw material compounds or derivatives thereof for the aimed compound and can be reutilized effectively.
Owner:MITSUI TAKEDA CHEM INC
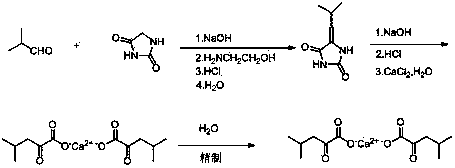
Environment-friendly technology for preparing ketoleucine calcium in aqueous phase
ActiveCN104058954AAvoid pollutionPollution is notCalcium/strontium/barium carbonatesOrganic compound preparationSolventHydrolysis
The invention relates to an environment-friendly technology for preparing ketoleucine calcium in an aqueous phase. Isobutylidene hydantoin and a calcium chloride aqueous solution serve as raw materials, and water serves as a solvent. The environment-friendly technology comprises the steps that heating reflux hydrolysis is carried out on the isobutylidene hydantoin in water and industrial liquid alkali, the calcium chloride aqueous solution is dropwise added, the mixed solution is filtered, filtered liquid is collected and cooled, hydrochloric acid acidification is carried out, vacuum nitrogen displacement is carried out, caustic soda liquid is added for alkalization, cooling is carried out, the calcium chloride aqueous solution is dropwise added, calcium salt is obtained, cooling is carried out, a crude product is obtained through filtering, the crude product is refined in purification water, and a ketoleucine calcium refined product is obtained. The environment-friendly technology for preparing the ketoleucine calcium in the aqueous phase has the advantages of being easy to operate, simple in steps, high in yield coefficient, good in product quality, environmentally friendly and the like. According to the environment-friendly technology for preparing the ketoleucine calcium in the aqueous phase, the environment-friendly water is adopted as the solvent completely, the requirement of green chemistry development is met, the pollution problem is solved from the source, and the environment-friendly technology is suitable for industrialized mass production.
Owner:SHAOXING MINSHENG PHARMA
Processes for purifying acetic anhydride
ActiveUS8759576B2Organic compound preparationPreparation from carboxylic acid amidesAcetic acidAcetic anhydride
In one embodiment, the invention is to a process for purifying acetic anhydride. The process includes the steps of feeding a liquid crude acetic anhydride stream directly to a distillation column and separating the liquid crude acetic anhydride stream to produce a light ends stream, a sidedraw and a residue stream. The sidedraw comprises substantially pure acetic anhydride product. The distillation column is operated at a pressure less than 101 kPa. The substantially pure acetic anhydride product comprises greater than 98 wt. % acetic anhydride, has a permanganate time of greater than 10 minutes, and has an APHA color of less than 10.
Owner:CELANESE INT CORP
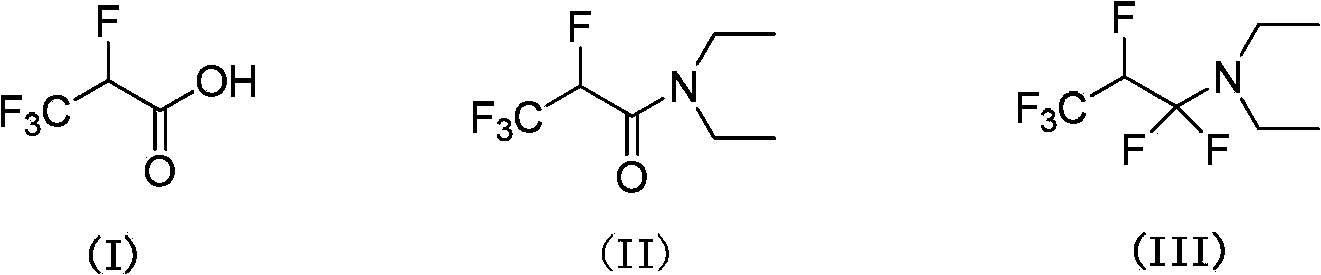
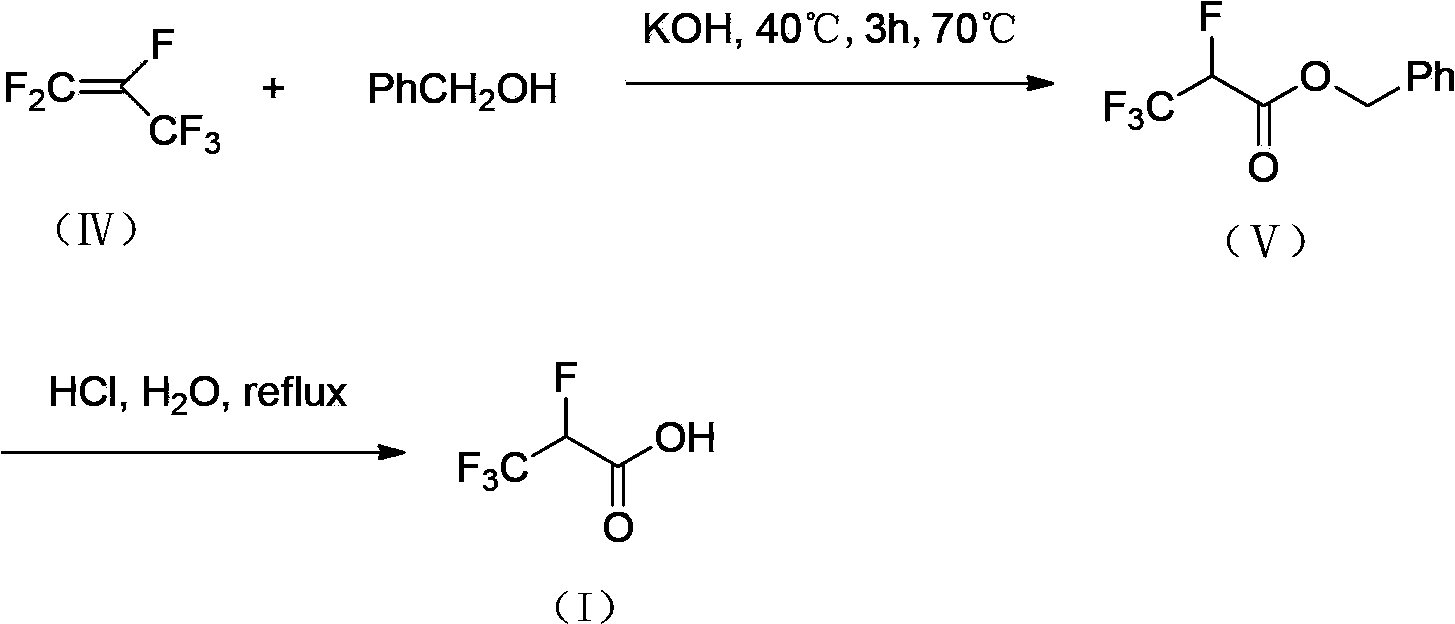
2,3,3,3-tetrafluoro propionic acid (i) synthesis method
InactiveCN103508875ASimple and fast operationLow production costPreparation from carboxylic acid amidesPropanoic acidSynthesis methods
Owner:赵磊 +1
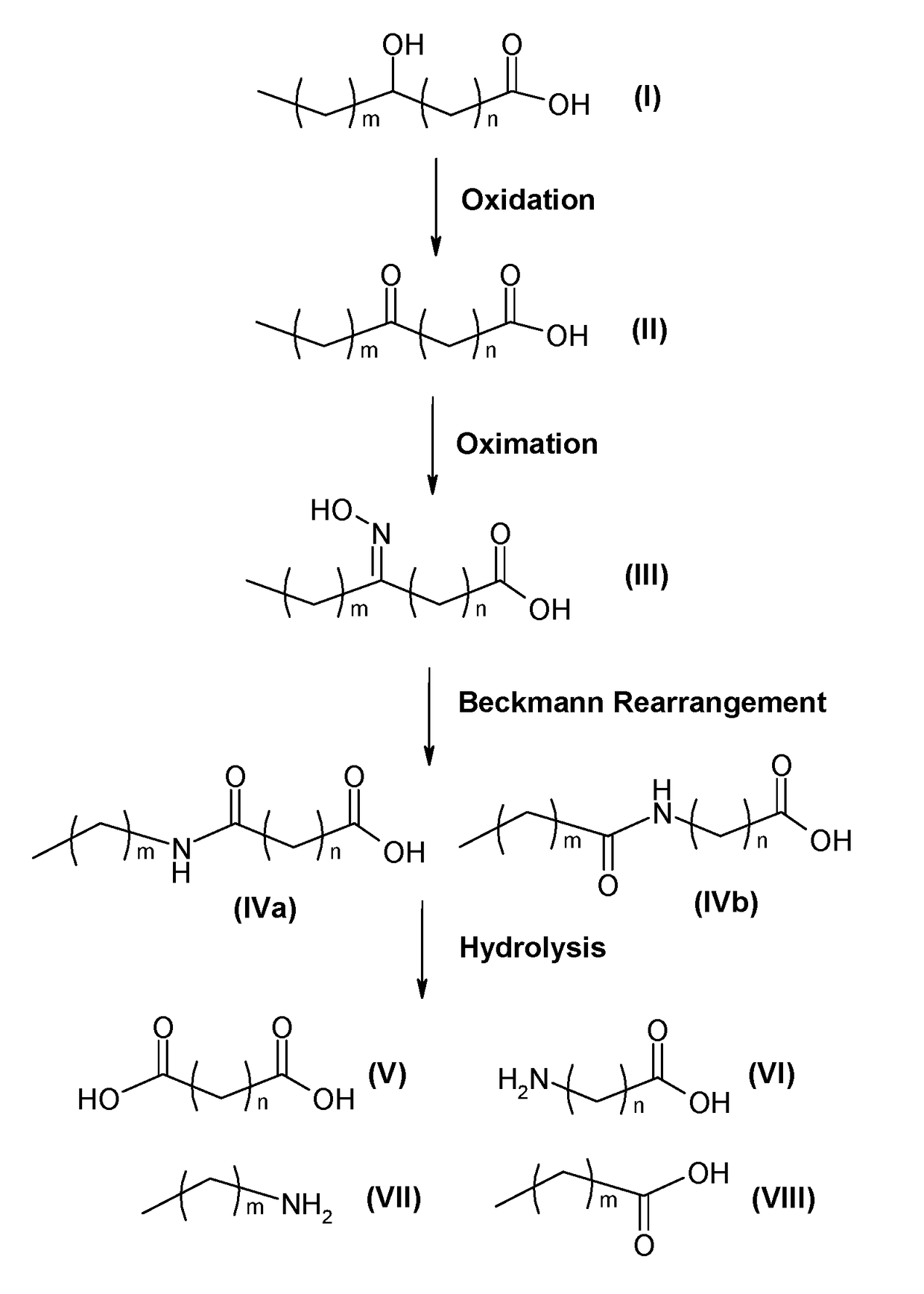
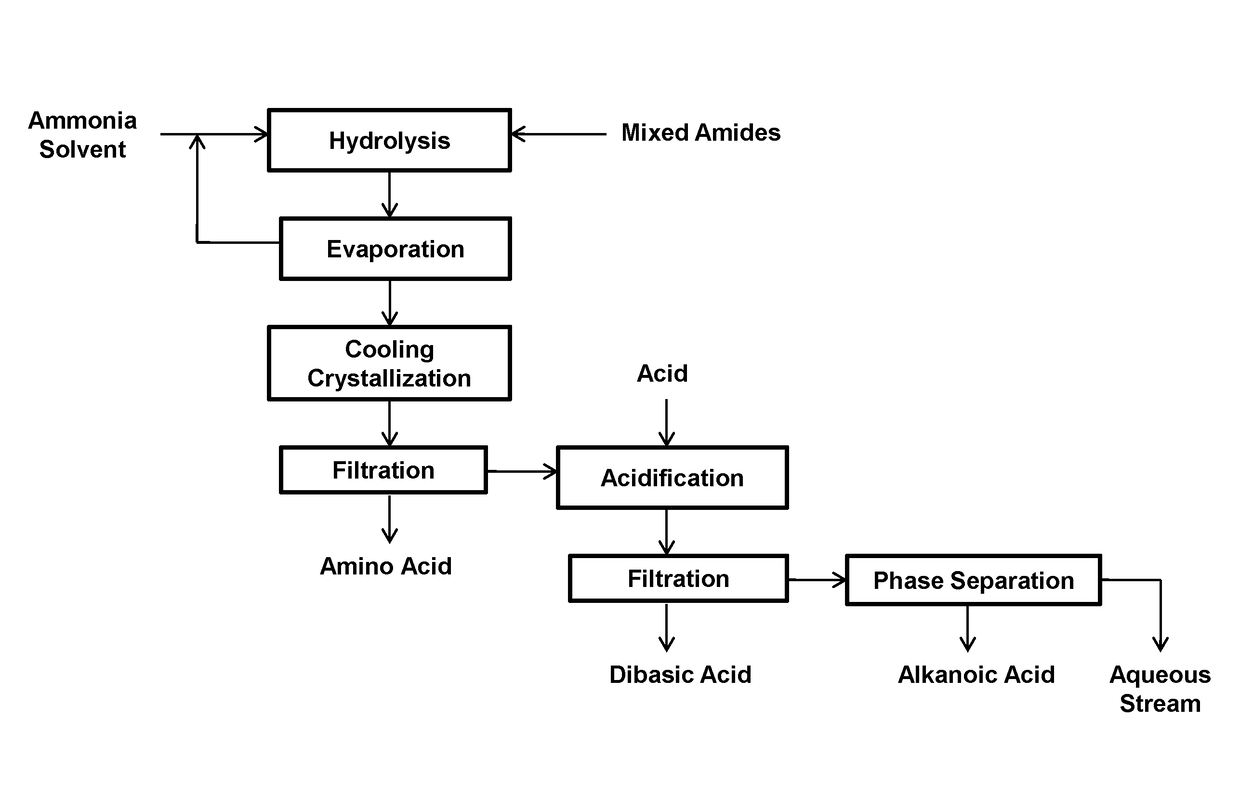
Process for producing long chain amino acids and dibasic acids
ActiveUS10065921B1Organic compound preparationCarboxylic acid amides preparationBeckmann rearrangementIodo fatty acid
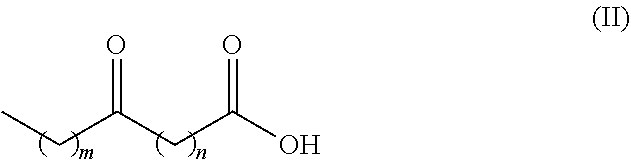
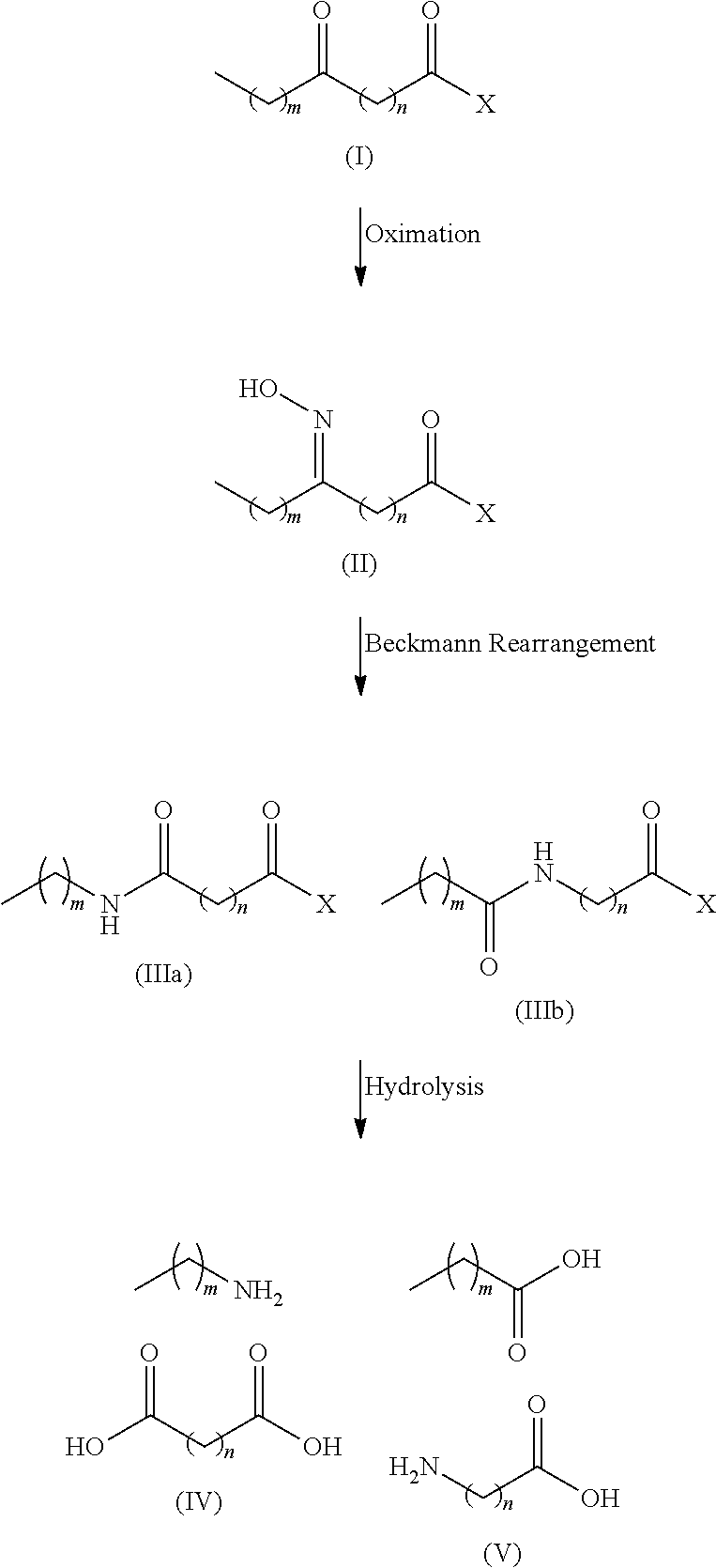
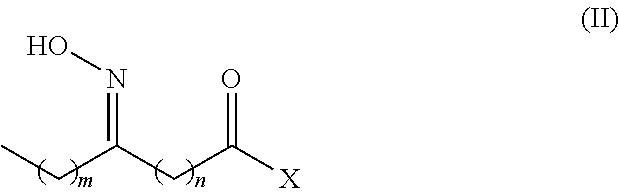
There is disclosed a process for the production of long chain amino acid and long chain dibasic acid, comprising: (1) reacting long chain keto fatty acid with hydroxylamine or subjecting keto fatty acid to an ammoximation reaction to yield an oxime fatty acid; (2) subjecting the oxime fatty acid to the Beckmann rearrangement to yield a mixture of two amide fatty acids; (3) hydrolyzing the mixed amide fatty acids to produce long chain amino acid, long chain dibasic acid, short chain alkylamine, and alkanoic acid.
Owner:VITAWORKS IP LLC
Method for preparing 2,3,3,3-tetrafluoropropene
InactiveCN107986938ARealize resource utilizationRaw materials are easy to getOrganic compound preparationHydroxy compound preparationReagentFree system
The invention relates to a method for preparing 2,3,3,3-tetrafluoropropene. The method comprises the following steps: (1) by taking a by-product N,N-diethyl-2,3,3,3-tetrafluoropropylamide of an Ishikawa's reagent as a raw material, hydrolyzing under acidic or alkaline conditions to obtain 2,3,3,3-tetrafluoropropionic acid; (2) contacting the 2,3,3,3-tetrafluoropropionic acid with a reducing agentin the presence of a catalyst or in a catalyst-free system so as to obtain 2,3,3,3-tetrafluoropropanol; and (3) reacting the 2,3,3,3-tetrafluoropropanol in the presence of a dehydrating agent, therebyobtaining the 2,3,3,3-tetrafluoropropene. The method disclosed by the invention is simple in operation, mild in conditions and low in equipment requirements, recycling of the by-product is realized,and industrialized application is easily realized.
Owner:HANGZHOU NORMAL UNIVERSITY +1
Process for recovering valued compounds from a stream derived from purification of methyl methacrylate
ActiveCN102030627AOrganic compound preparationCarboxylic acid esters preparation2-methylpropeneOrganic chemistry
The present invention provides a method for extractive recovery and conversion of selected compounds, such as methacrylic acid (MAA) and 2-methacrylamide (MAM), from a stream derived from purification of methyl methacrylate (MMA) or methacrylic acid (MAA) produced via a conventional ACH route process.
Owner:ROHM & HAAS CO
Process for the co-production of long chain amino acids and dibasic acids
ActiveUS20190016668A1Delayed reaction timeReduce the amount of solutionOrganic compound preparationCarboxylic acid amides preparationBeckmann rearrangementHydroxylamine
There is disclosed a process for the co-production of long chain ω-amino acid and long chain dibasic acid, comprising: (1) reacting long chain ketoacid derivative with hydroxylamine or subjecting ketoacid derivative to an ammoximation to yield oxime derivative; (2) subjecting oxime derivative to Beckmann rearrangement to yield a mixture of mixed amide derivatives; (3) hydrolyzing the mixed amide derivatives to produce long chain ω-amino acid and long chain dibasic acid.
Owner:VITAWORKS IP LLC
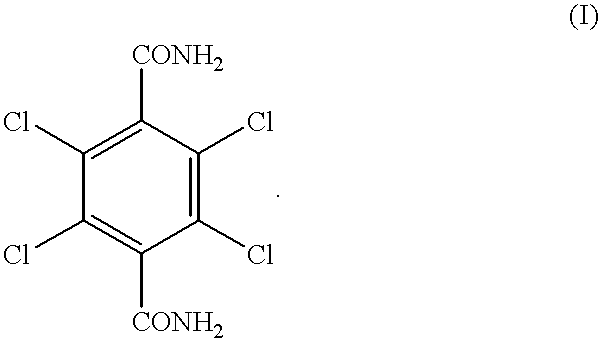
Process for producing 2, 3, 5, 6-tetrachloro-1, 4-benzenedicarboxylic acid
InactiveUS20010025121A1High yieldEasy to operateBiocidePhysical/chemical process catalystsAcidity functionHydrolysis
A process for producing 2,3,5,6-tetrachloro-1,4-benzenedicarboxylic acid, comprising heating 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide together with sulfuric acid exhibiting an acidity function (-Ho) of 10.27 to 14.44 in the presence of water contained in an amount smaller than a stoichiometric value of hydrolysis at 110 to 190° C. This process efficiently produces 2,3,5,6-tetrachloro-1,4-benzenedicarboxylic acid which is a precursor of dimethyl 2,3,5,6-tetrachloro-1,4-benzenedicarboxylate useful as a herbicide.
Owner:SDS BIOTECH CO LTD
V-P-Si composite oxide catalyst precursor used for producing maleic anhydride from butance
InactiveUS7547655B2Increase productionEasy to carryOrganic compound preparationHeterogenous catalyst chemical elementsIsobutanolPhosphoric acid
The present invention provides a catalyst precursor for producing maleic anhydride by oxidizing butane. Said catalyst precursor is prepared by a process comprising partially reducing V+5 to V+4 in a mixture of alcohols consisting of isobutanol and benzyl alcohol in a volume ratio of 2.5-5.0, then adding a phosphoric oxy-acid and an alkylsilicon in turn. The catalyst precursor prepared according to said process has a small pore volume, a relatively high bulk density in an appropriate pore size distribution.
Owner:BEIJING HUAI YING MALEIC ANHYDRIDE SCI & TECH CO LTD
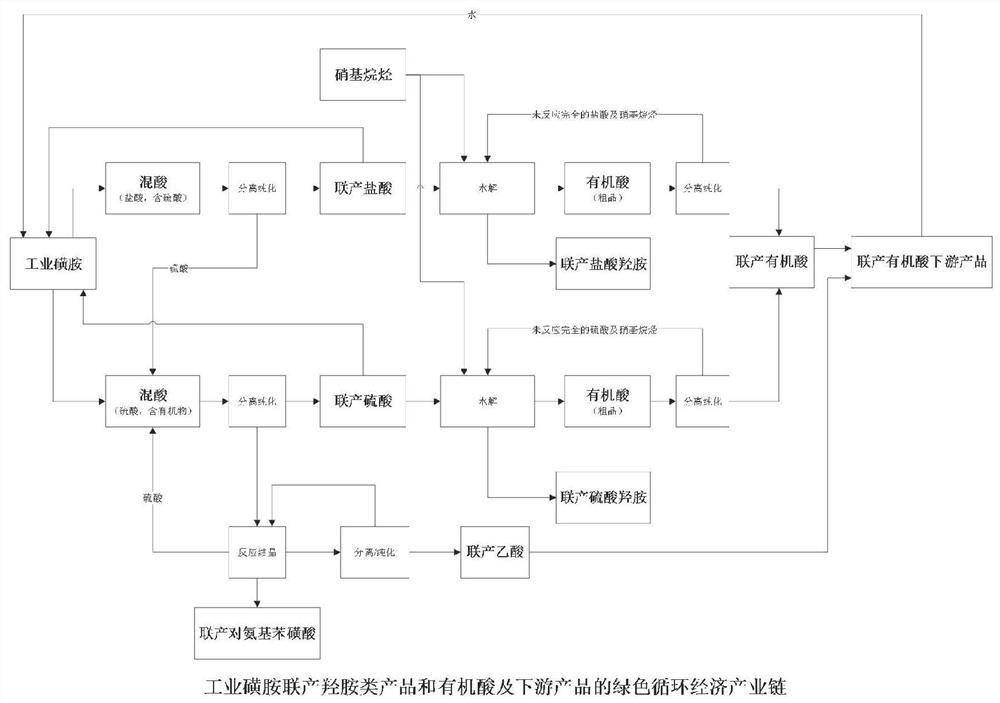
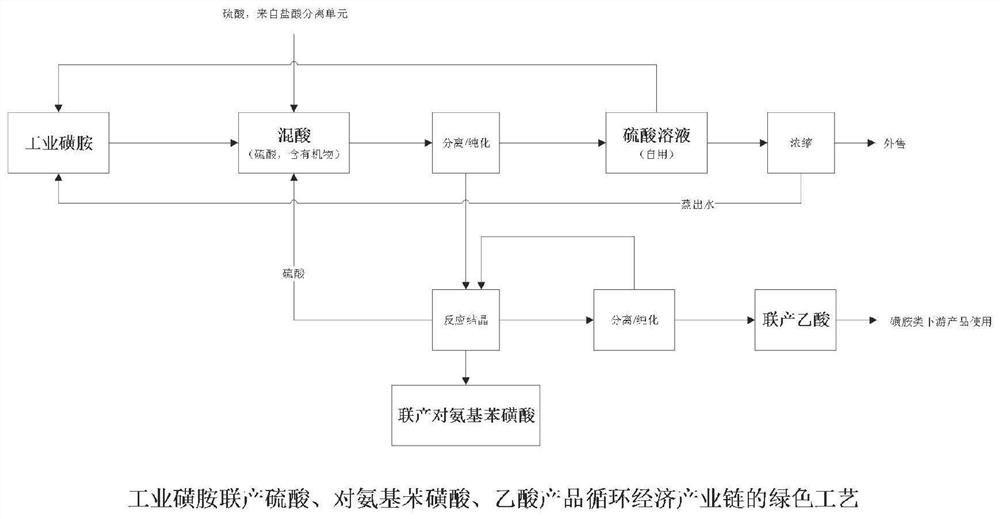
Green process of circular economy industry chain for co-production of industrial sulfanilamide and hydroxylamine products and organic acid downstream products
ActiveCN113321578ASolve the technical problems of separation and purificationHigh economic valueChlorine/hydrogen-chloride purificationOrganic compound preparationAlkaneNitroalkane
The invention provides a green process of a circular economy industry chain for co-production of industrial sulfanilamide and hydroxylamine products and organic acid downstream products. The process comprises the following steps that hydrochloric acid mixed acid is separated and purified to obtain sulfuric acid and co-produced hydrochloric acid, and the obtained sulfuric acid enters a sulfuric acid mixed acid treatment route; partial co-produced hydrochloric acid and nitroalkane are subjected to a hydrolysis reaction to obtain organic mixed acid and co-produced hydroxylamine hydrochloride, and the organic mixed acid is separated and purified to obtain co-produced organic acid; the sulfuric acid mixed acid is separated and purified to obtain organic matters and co-produced sulfuric acid; the organic matters are subjected to reactive crystallization to co-produce p-aminobenzene sulfonic acid; and partial co-production sulfuric acid and nitroalkane are subjected to a hydrolysis reaction to obtain a co-production hydroxylamine sulfate product and organic mixed acid, and the organic mixed acid is separated to obtain co-produced organic acid. According to the process, a green circular economy industrial chain of industrial sulfanilamide is realized, and meanwhile, a green circular economy industrial chain of hydroxylamine products and organic acid downstream products can be co-produced.
Owner:湖南吴赣药业有限公司
Method for producing alpha-hydroxycarboxylic acid esters
InactiveCN103687841AExtended service lifeLow selectivityOrganic compound preparationCarboxylic acid esters preparationAlcoholGas phase
The invention relates to a continuous method for producing alpha-hydroxycarboxylic acid esters, at least one alpha-hydroxycarboxylic acid amide, which is present in the liquid phase, being reacted with an alcohol in the presence of a catalyst. The method is characterized in that the obtained alpha-hydroxycarboxylic acid esters are at least partially removed from the reaction mixture by means of the gas phase.
Owner:EVONIK ROEHM GMBH
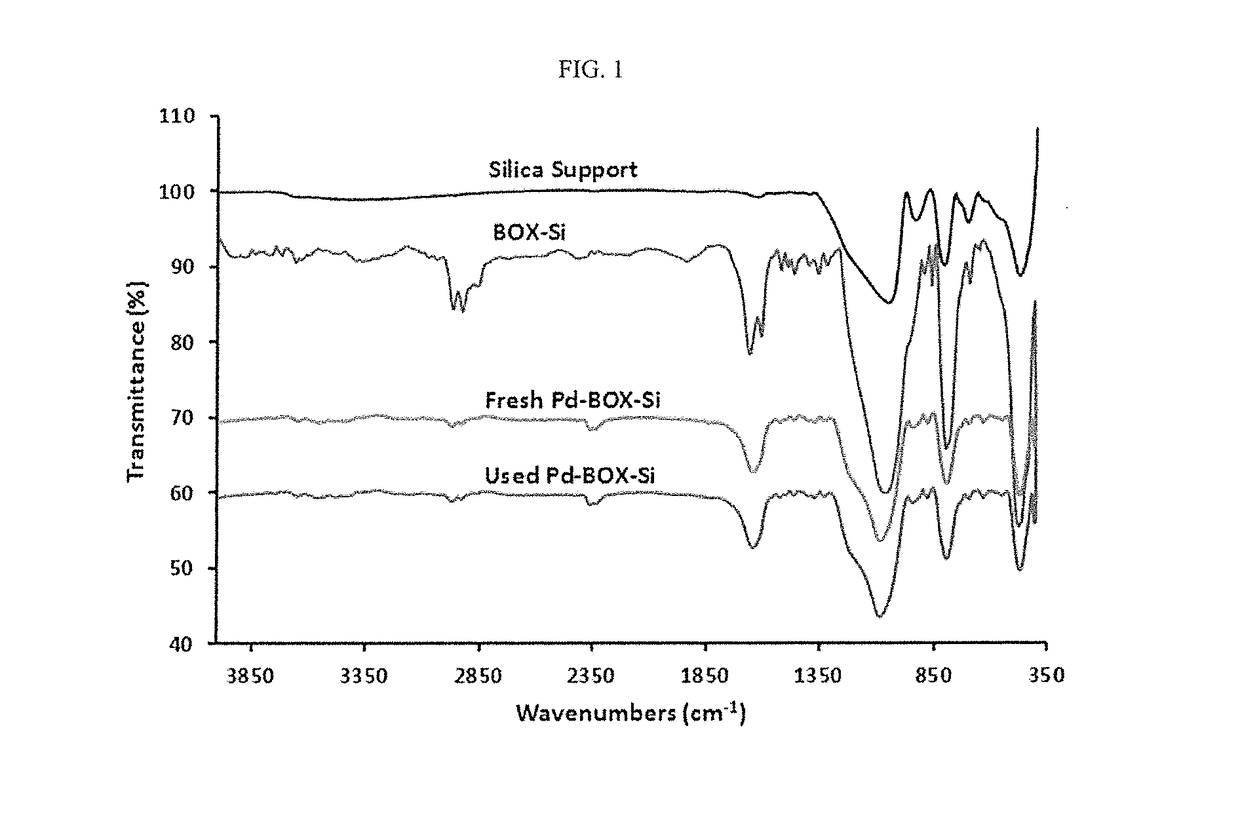
Solid-supported palladium(II) complex for catalyzing mizoroki-heck coupling reactions and a method thereof
InactiveUS20180065117A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCouplingCoupling reaction
A solid-supported palladium(II) complex which catalyzes the Mizoroki-Heck coupling reaction efficiently and a method of employing the solid-supported palladium(II) complex to synthesize cinnamic acid and derivatives thereof. The solid-supported palladium(II) complex is also stable and can be recycled without significantly losing catalytic activity.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
V-p-ci composite oxide catalyst precursor used for producing maleic anhydride from butance
InactiveUS20060241310A1Increase productionEasy to carryOrganic compound preparationHeterogenous catalyst chemical elementsIsobutanolPhosphoric acid
The present invention provides a catalyst precursor for producing maleic anhydride by oxidizing butane. Said catalyst precursor is prepared by a process comprising partially reducing V+5 to V+4 in a mixture of alcohols consisting of isobutanol and benzyl alcohol in a volume ratio of 2.5-5.0, then adding a phosphoric oxy-acid and an alkylsilicon in turn. The catalyst precursor prepared according to said process has a small pore volume, a relatively high bulk density in an appropriate pore size distribution.
Owner:BEIJING HUAI YING MALEIC ANHYDRIDE SCI & TECH CO LTD
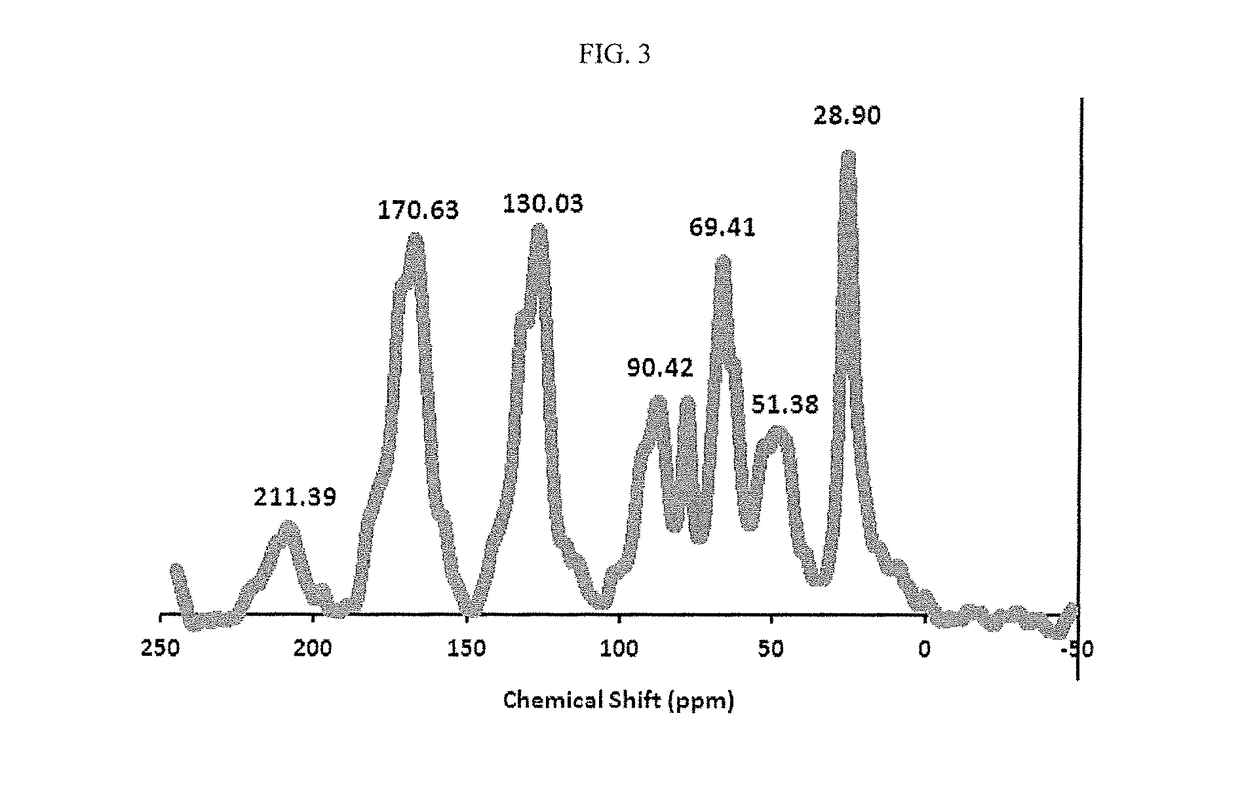
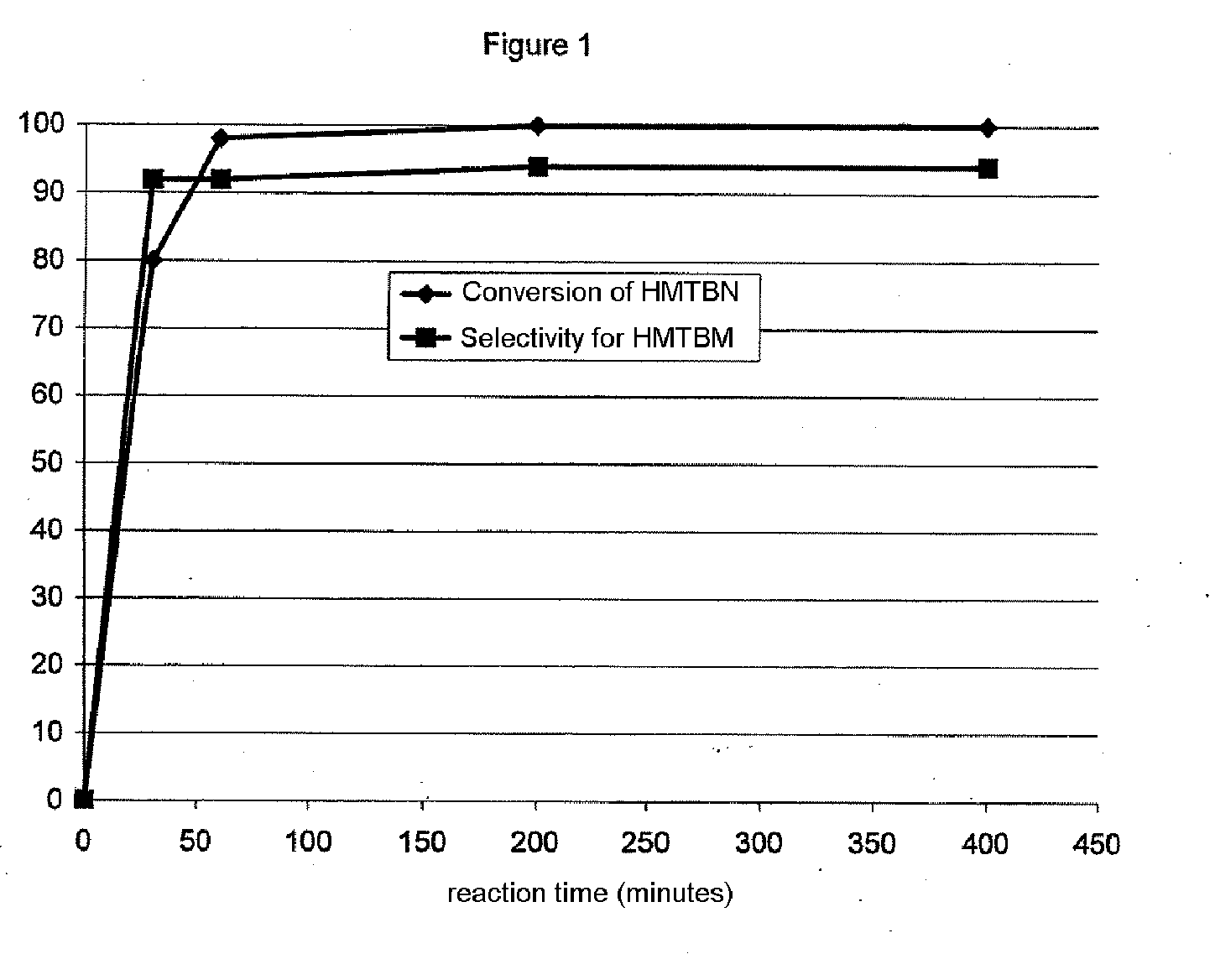
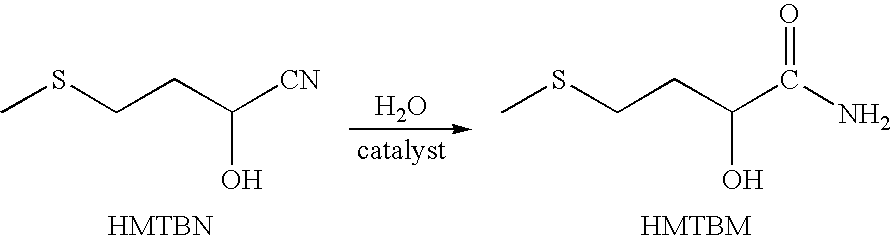
Method for the catalytic conversion of 2-hydroxy-4-methylthiobutanenitrile (HMTBN) into 2-hydroxy-4-methylthiobutanamide (HMTBM)
InactiveUS20100197965A1Resist formationNotOrganic compound preparationCatalyst activation/preparationActive phaseOrganic chemistry
This process is carried out in the presence of a solid catalyst comprising an active phase. The catalyst is formulated and the conversion is carried out in a medium essentially free of strong mineral acid.
Owner:ADISSEO IRELAND LTD
Process for synthesizing 2, 3, 3, 3-tetrafluoropropionic acid
ActiveCN103254058AReduce processing costsCheap and easy to getPreparation from carboxylic acid amidesChemical synthesisReaction speed
The invention relates to a chemical synthesis process, and particularly relates to a process for synthesizing 2, 3, 3, 3-tetrafluoropropionic acid. According to the process, N, N-diethyl-2, 3, 3, 3-tetrafluoropropionamide as the starting material is subjected to a hydrolysis reaction under the acidic condition by using a catalyst; the side product N, N-diethyl-2, 3, 3, 3-tetrafluoropropionamide obtained by the fluorine substitution reaction of N, N-diethyl-1, 1, 2, 3, 3, 3-tetrafluoropropionamide is effectively utilized to obtain high-purity 2, 3, 3, 3-tetrafluoropropionic acid through the simple, easily-operated and easy separation and extraction process, the addition of the catalyst greatly accelerates the reaction speed and improves the product yield which reaches 96 percent, the benefit is built, the waste liquid treatment cost is reduced, and the environment is protected; the raw materials in the invention are low in price and easily obtained, the reaction conditions are mild, the reaction is speedy, the process is simple, easily operated and easily treated; and the process is beneficial for achievement of industrialization.
Owner:京山瑞生制药有限公司
Method for producing hexane diacid and hexanediamine by using nylon-66 disaggregation
InactiveCN101423478AQuick responseIncrease productivityOrganic compound preparationPlastic recyclingHexamethylenediamineAcid hydrolysis
The invention relates to a method for producing hexanedioic acid and hexamethylene diamine by using nylon-66 to depolymerize, which is characterized by comprising the following steps: adding formylic acid and water into a reaction kettle, heating the mixture and adding the nylon-66 into the mixture at the same time, dissolving the nylon-66, adding sulphuric acid into the mixture, making the mixture react for 4 to 10 hours under the condition of keeping pressure intensity between 0.1 and 0.5MPa, so as to obtain acid hydrolysis solution; filtering obtained wet CaSO4.2H2O, and then obtaining dry CaSO4.2H2O and hexamethylene diamine solution by adopting a drying method; and adding Na3PO4 in the production process to prevent CaSO4 from scaling. By adopting the technique, the method can improve production efficiency, reduces even does not use phosphoric acid or phosphate, reduces production cost, improves recovery rate of the hexamethylene diamine, eliminates scaling in the production process, and reduces risk in the running process of equipment.
Owner:NINGBO UNIV
Substituted heterocyclic compounds for treating multidrug resistance
InactiveUS20020128269A1Avoid developmentOrganic active ingredientsPreparation from carboxylic acid amidesHeterocyclic compound
Owner:THE PROCTER & GAMBLE COMPANY
Precursor of V-P-Si composite oxides catalyst for preparing maleic anhydride by oxidizing butane
InactiveCN1520927AOrganic compound preparationHeterogenous catalyst chemical elementsO-Phosphoric AcidPtru catalyst
The present invention provides one kind of catalyst precursor for preparing cis-butenedioic anhydride via catalyzed butane oxidation. The catalyst precursor is prepared through reducing at least partially V+5 into V+4 and adding phosphoric acid and alkyl silicide successively. The catalyst precursor has relatively small porocity, relatively great bulk density and proper pore size distribution.
Owner:NEW TIANJIN T & D
Process for producing long chain amino acids and dibasic acids
ActiveUS10053416B1Organic compound preparationCarboxylic acid amide separation/purificationEvaporationSolvent
There is disclosed a process for the separation of long chain amino acid and long chain dibasic acid, comprising: (1) mixing the mixed amide derivatives with an aqueous solution of ammonia or ammonium hydroxide in the presence or absence of solvent or catalyst; (2) subjecting the solution or suspension of step (1) to an hydrolysis reaction; and (3) recovering excess ammonia and solvent in the presence of solvent in step (1) by evaporation to provide a mixture of long chain amino acid and alkylamine or ammonium salts of long chain dibasic acid and alkanoic acid.
Owner:VITAWORKS IP LLC
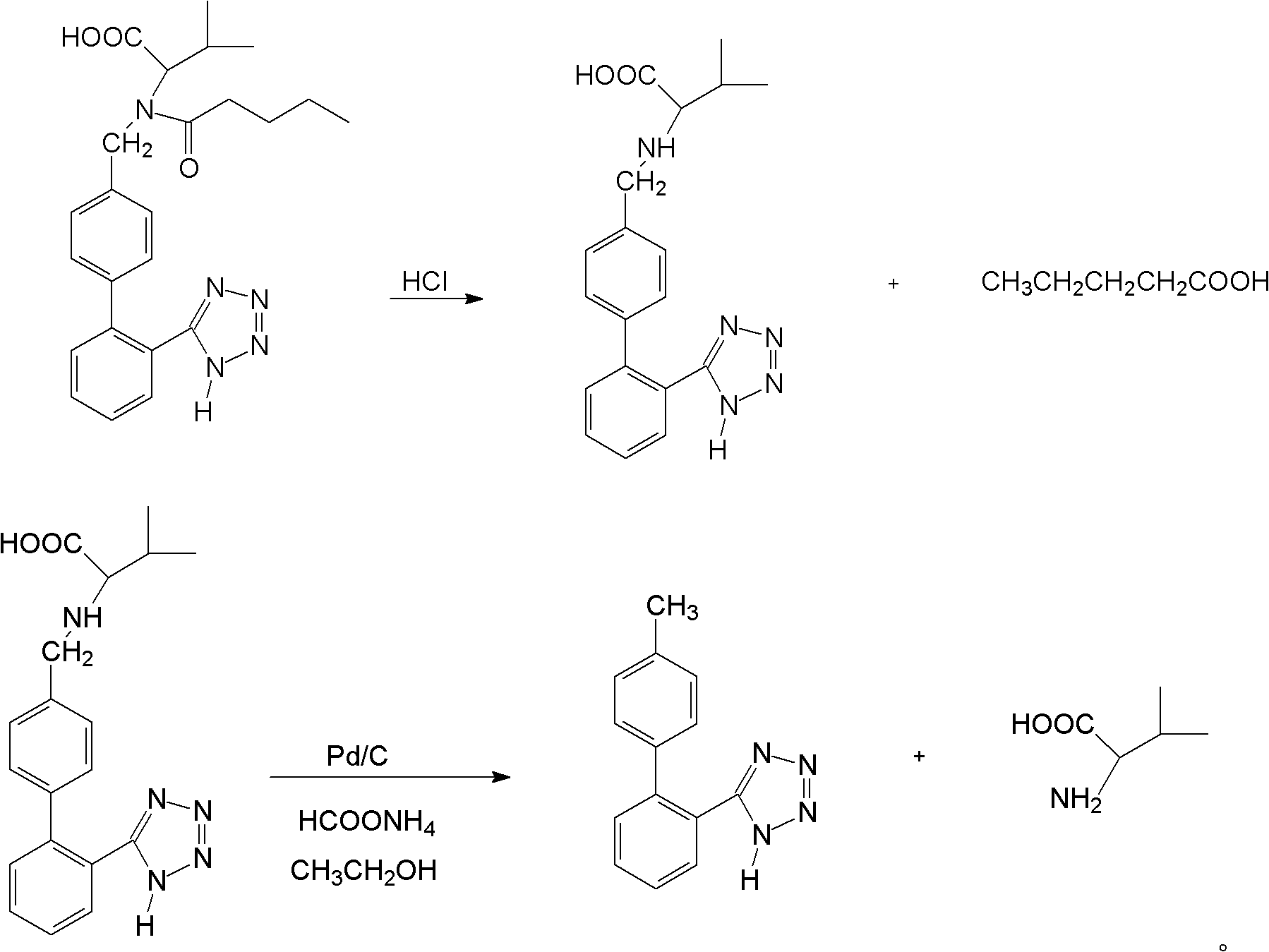
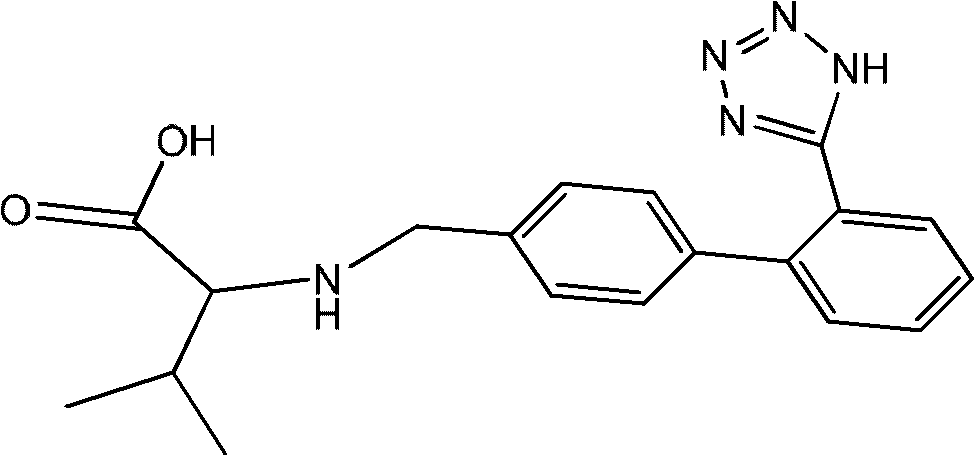
Method for recovering valsartan racemate
ActiveCN102351804AEasy to purifyHigh selectivityOrganic compound preparationPreparation from carboxylic acid amidesAlcoholLosartan
The invention discloses a method for recovering valsartan racemate, which comprises the following steps that: (1) pentanoic acid and valsartan depentanized acyl products are obtained through hydrolysis reaction of valsartan racemate in acid aqueous solution; and (2) in alcohol solvents, the valsartan depentanized acyl products take reduction reaction under the effect of reducing agents and catalysts to obtain valine and 4'-methyl phenylbenzene-2-hydroxyphenyl tetrazole. The method has the advantages that the steps are simple and convenient, the method is applicable to industrial production, and the 4'-methyl phenylbenzene-2- hydroxyphenyl tetrazole, the valine and the pentanoic acid can be recovered, wherein the 4'-methyl phenylbenzene-2-tetrazole is an important raw material for synthesizing losartan important intermediate N-(trityl)-5-(4'-bromine methyl phenylbenzene)-2-hydroxyphenyl) tetrazole.
Owner:浙江新赛科药业有限公司
Method for joint production of methacrylamide, methyl methacrylate and methacrylic acid
ActiveCN103254091AReduce lossesHigh yieldOrganic compound preparationCarboxylic acid esters preparationAcetone cyanohydrinHydrolysis
The invention discloses a method for joint production of methacrylamide, methyl methacrylate and methacrylic acid. Methacrylamide sulfate is prepared from acetone cyanohydrin which is taken as a major raw material through concentrated sulfuric acid hydrolysis, dehydration and other effects; the methacrylamide sulfate is neutralized through ammonia gas or ammonia water, and at least one part of neutralized mother liquor circulates to an esterification step of producing methyl methacrylate and a hydrolysis step of producing methacrylic acid, so as to produce methyl methacrylate and methacrylic acid. The joint production method can realize the complete reuse of the methacrylamide in the mother liquor and convert the methacrylamide into the methyl methacrylate and the methacrylic acid, so as to reduce the loss of the methacrylamide, improve the yields of the methyl methacrylate and the methacrylic acid, and reduce the discharge of wastewater; and besides, the neutralized mother liquor can be extracted to avoid the accumulation of impurities in a process of purifying the methacrylamide.
Owner:CHONGQING UNISPLENDOUR CHEM
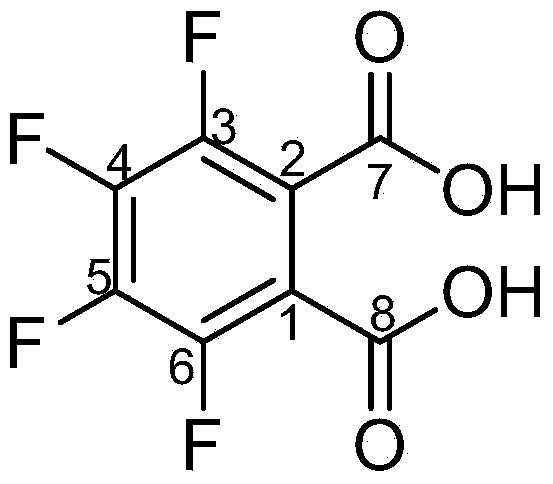
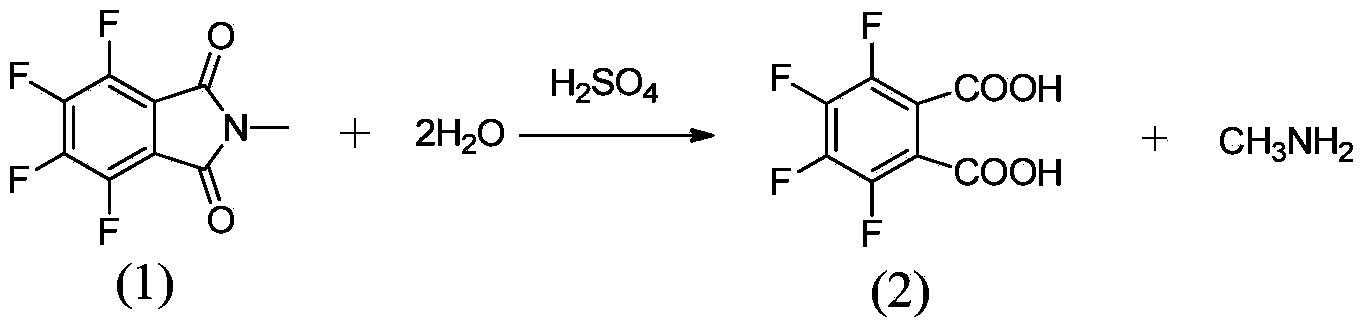
Method for preparing 3,4,5,6-tetrafluorophthalic acid
InactiveCN104072358AAvoid it happening againRelieve pressureOrganic compound preparationPreparation from carboxylic acid amidesCatalytic hydrolysisBy-product
The invention provides a method for preparing a 3,4,5,6-tetrafluorophthalic acid. N-methyl tetrafluorophthalimide is subjected to catalytic hydrolysis by a catalyst to generate the 3,4,5,6-tetrafluorophthalic acid at the pressure of 0.5-3.0kg / cm<2> and the temperature of 106-140 DEG C. By adopting the technical scheme disclosed by the invention, waste sulfuric acid is avoided, the reacted by-product methylamine can be applied to recovering and preparing the raw material N-methyl tetrafluorophthalimide, the pressure to the environment is greatly relieved, and the production cost is also significantly reduced when the yield of the product is improved.
Owner:JIANGSU SHAXING CHEM
Method for manufacturing aromatic nitrile compound
PendingCN112041298ASafe and cheap to manufactureOrganic compound preparationCarboxylic acid amides preparationPtru catalystOrganosolv
Owner:API CO LTD
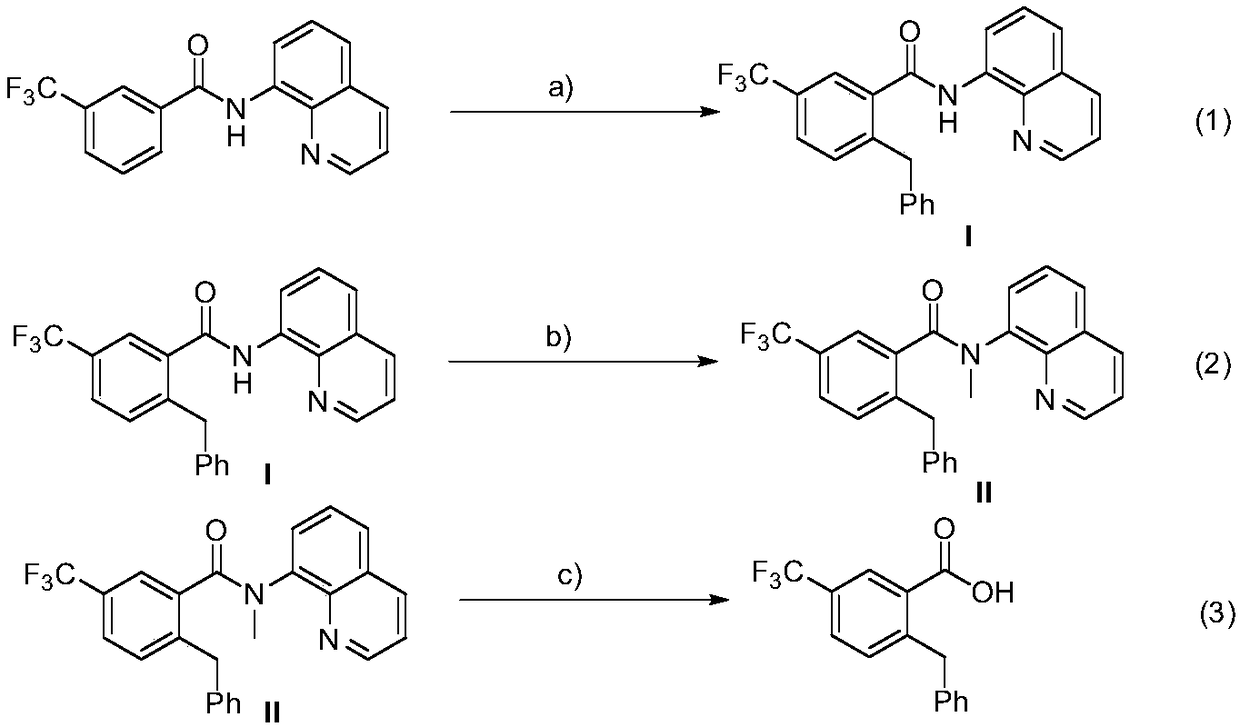
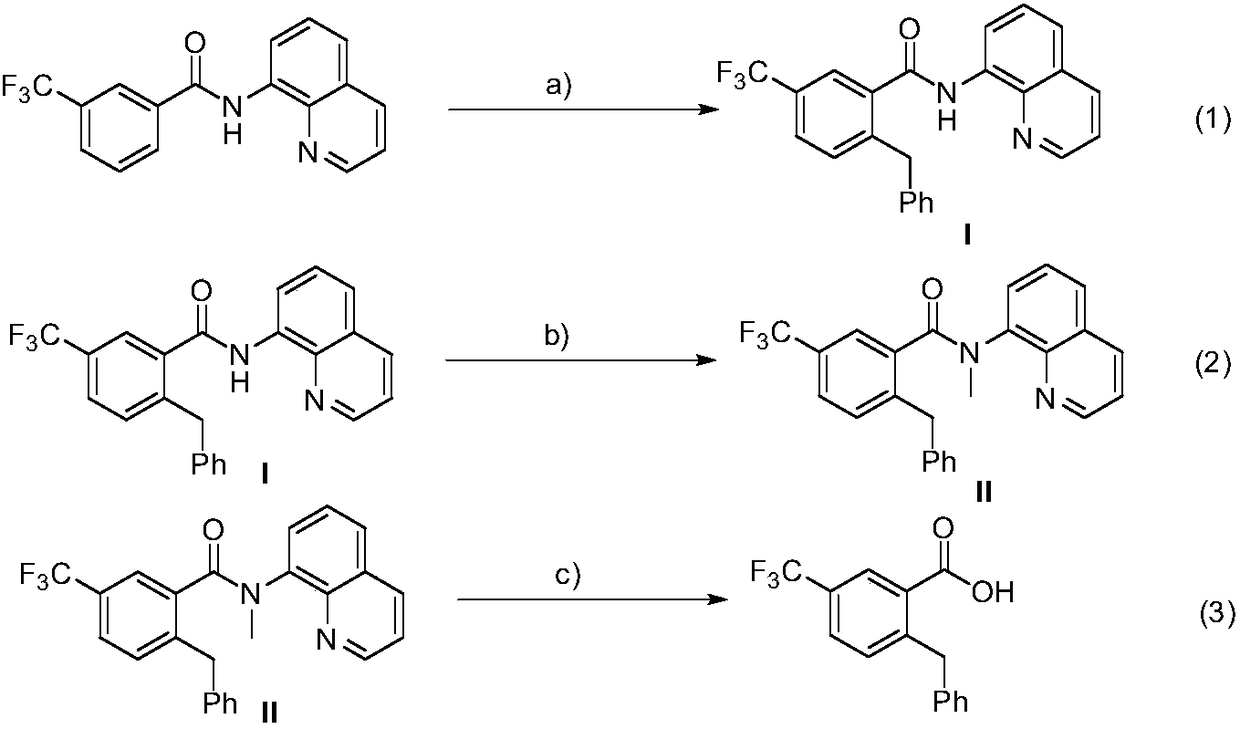
Method for preparation of 2-benzyl-5-(trifluoromethyl)benzoic acid
ActiveCN108752186ALow priceHigh yieldPreparation from carboxylic acid amidesBenzoic acidOrganic synthesis
The invention belongs the technical field of organic synthesis, and particularly relates to a method for preparation of 2-benzyl-5-(trifluoromethyl)benzoic acid. The method comprises the steps: enabling N-(8-quinolyl)-3-(trifluoromethyl) benzamide as an original material to be subjected to three steps of reaction including benzylation, methylation, and hydrolysis and acidification, thereby obtaining 2-benzyl-5-(trifluoromethyl) benzoic acid. The benzylation reaction adopts cobalt diacetylacetonate to serve as a catalyst, use of alkali is not needed, and the catalysis system has the advantagesof cheap price and high yield of a target product. A small amount of water is added in the hydrolysis step, to promote hydrolysis of an intermediate, and shorten reaction time and obtain a higher yield of the target product.
Owner:安徽古尔特科技有限公司
Hydrolysis process of 2-methyl-4-amino-5-(formamidomethyl)pyrimidine
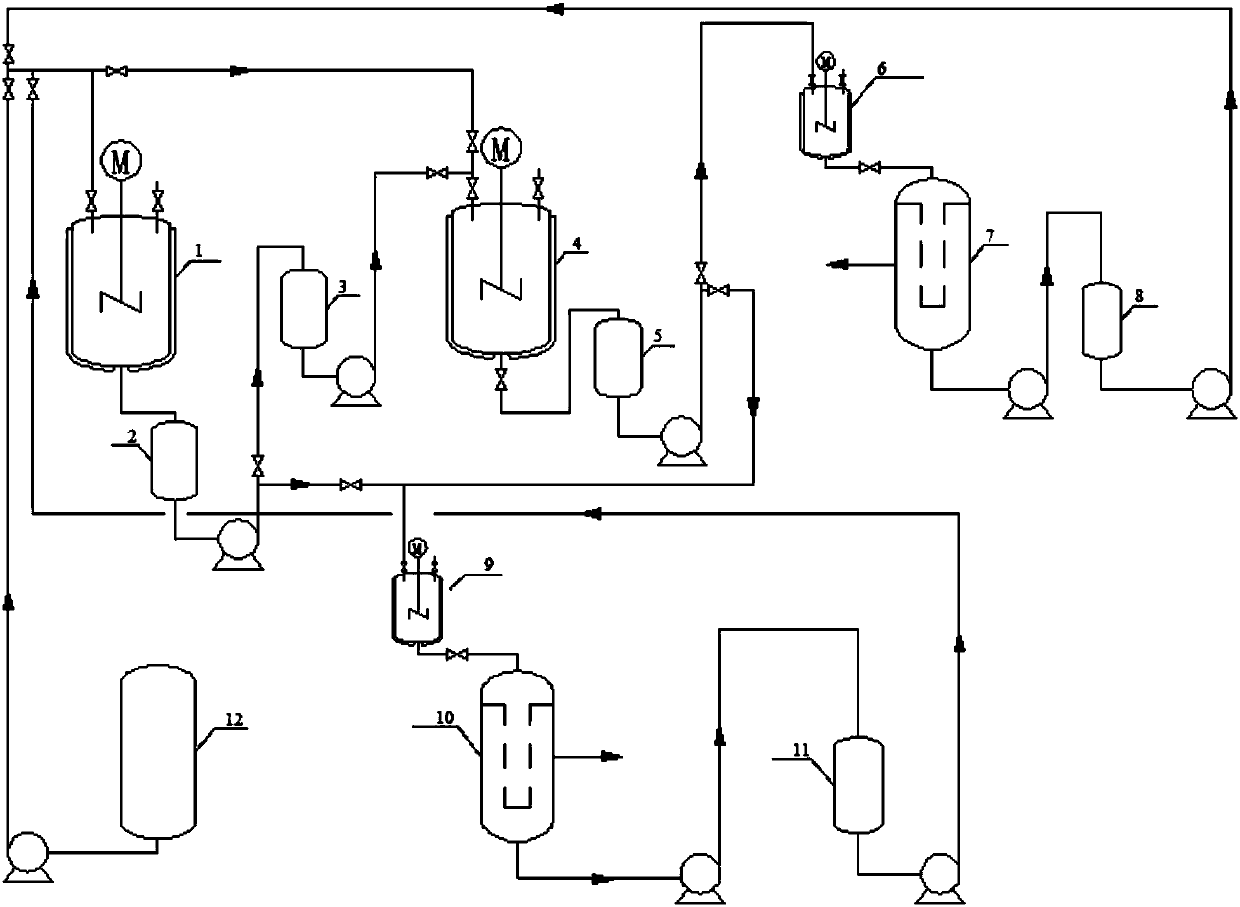
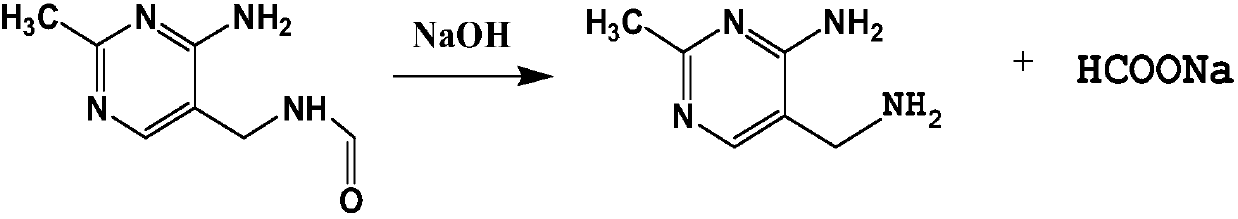
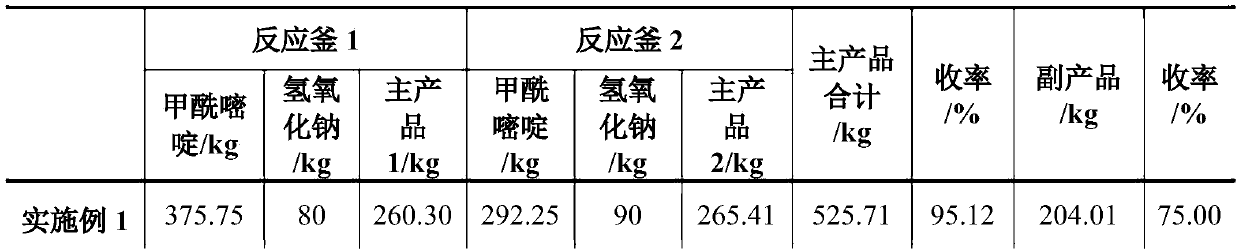
InactiveCN107602481AFully hydrolyzedReduce difficultyPreparation from carboxylic acid amidesCarboxylic compound separation/purificationWastewaterSolvent
The invention relates to a hydrolysis process of 2-methyl-4-amino-5-(formamidomethyl)pyrimidine for preparing 2-methyl-4-amino-5-(aminomethyl)pyrimidine. The process is a hydrolysis process of 2-methyl-4-amino-5-(formamidomethyl)pyrimidine and comprises the following steps that alternative excess reaction of the two raw materials is performed, the generated product is extracted by a solvent, theproduct is obtained by cooling precipitation, and the solvent is repeatedly used with water. Compared with the prior art, in the process, the 2-methyl-4-amino-5-(formamidomethyl)pyrimidine can be fully hydrolyzed, the product content is high, and the byproduct sodium formate can be separated out. In addition, no wastewater is generated in the process, and the solvent is not consumed.
Owner:CHANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com