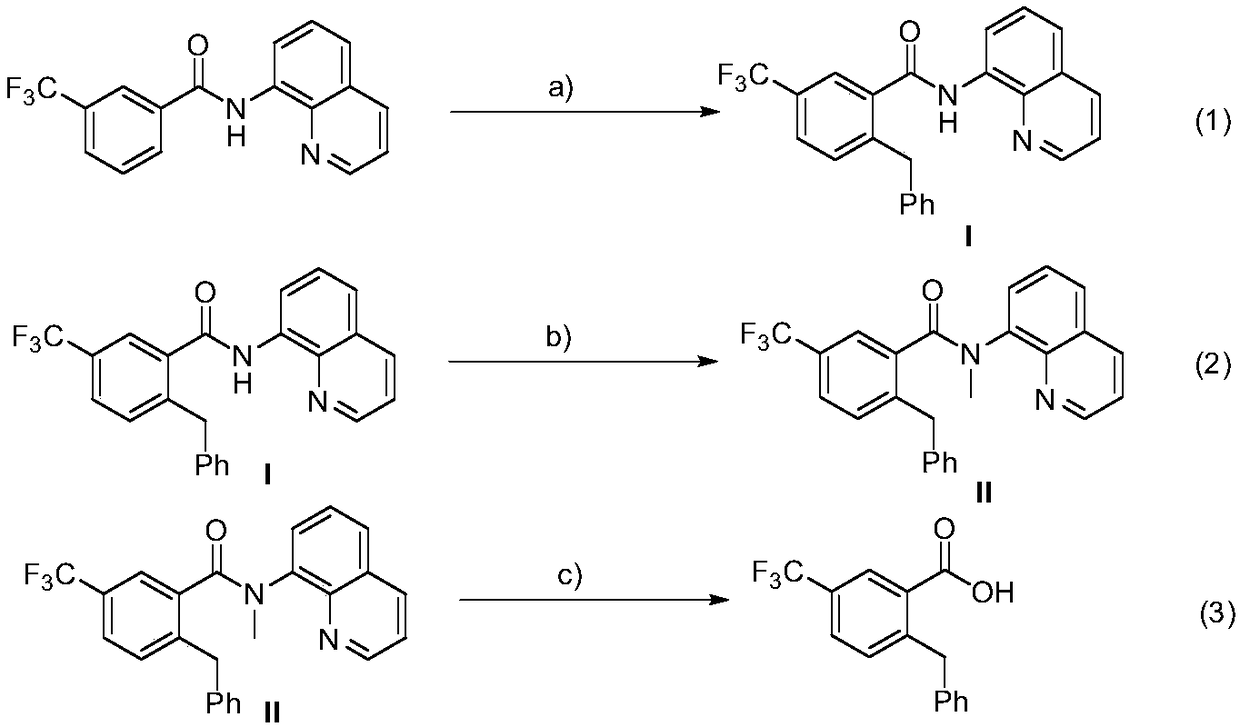
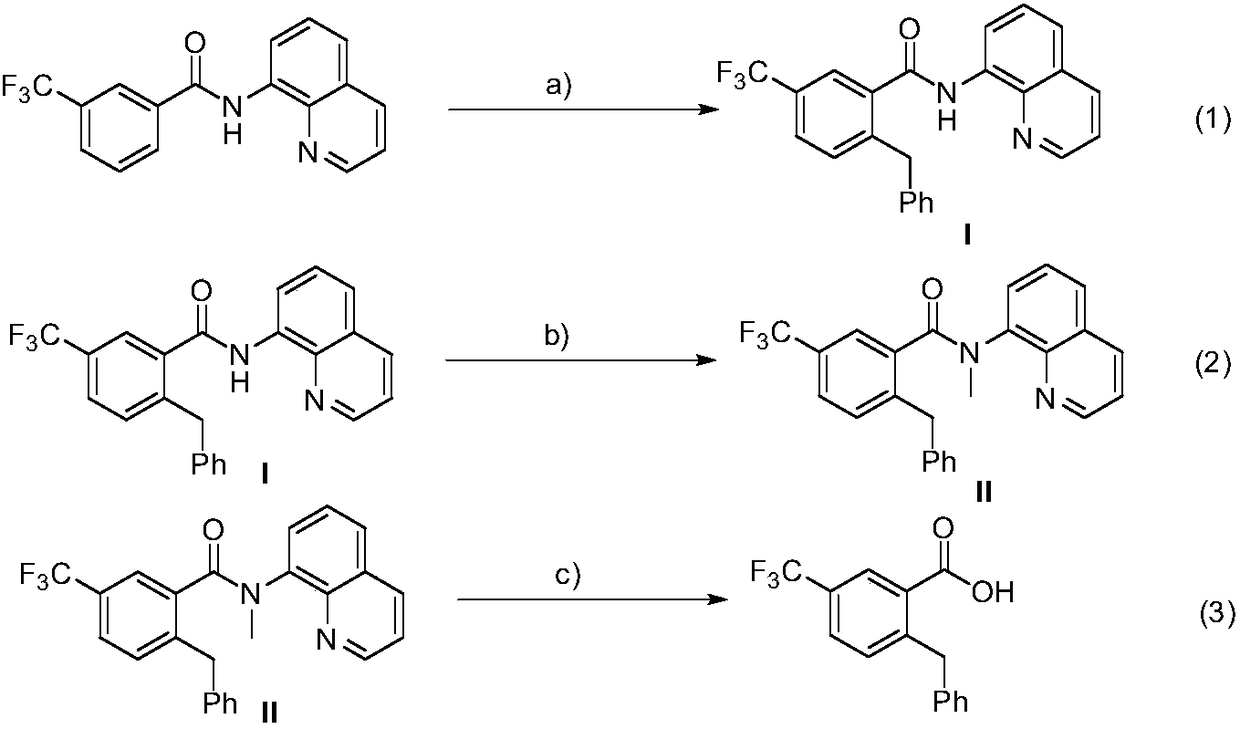
Method for preparation of 2-benzyl-5-(trifluoromethyl)benzoic acid
A technology of trifluoromethyl benzoic acid and trifluoromethyl, which is applied in the field of preparation of 2-benzyl-5-trifluoromethyl benzoic acid, can solve the problem of high reaction cost, harsh reaction conditions, unsuitable for industrial production, etc. problem, to achieve the effect of high yield and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Add 3-trifluoromethyl-N-(8-quinoline)benzamide (63.2mg, 0.2mmol), DTBP (58.5mg, 0.4mmol), and Co(acac )2 (14.2mg, 0.04mmol), then add 2mL of anhydrous toluene under nitrogen, heat the reaction to 130°C, and stir the reaction for 6 hours. After the reaction was completed, filter and wash with ethyl acetate, the filtrate was spin-dried, and the residual compound was separated by column chromatography on a silica gel column (petroleum ether / ethyl acetate=10 / 1~20 / 1) to obtain intermediate (I). Efficiency 87%, 1H NMR (500MHz, Chloroform-d) δ10.12(s, 1H), 8.86(d, J=6.4Hz, 1H), 8.65–8.60(m, 1H), 8.11(d, J=8.0 Hz,1H),7.85(s,1H),7.55–7.46(m,3H),7.36–7.35(m,1H),7.28(d,J=8.0Hz,1H),7.11–7.01(m,5H) ,4.25(s,2H).
[0025] (2) Add intermediate (I) to a round bottom flask, add 10mL of anhydrous tetrahydrofuran as a solvent, place the round bottom flask under ice bath conditions, then add 15 mg of sodium hydride in batches, stir for a period of time and slowly drop Add 85mg of met...
Embodiment 2
[0028] (1) Add 3-trifluoromethyl-N-(8-quinoline) benzamide (63.2mg, 0.2mmol), DTBP (87.6mg, 0.6mmol), and Co(acac )2 (14.2mg, 0.04mmol), then added 2mL of anhydrous toluene under nitrogen, heated the reaction to 120°C, and stirred for 8 hours. After the reaction was completed, filter and wash with ethyl acetate, the filtrate was spin-dried, and the residual compound was separated by column chromatography on a silica gel column (petroleum ether / ethyl acetate=10 / 1~20 / 1) to obtain intermediate (I). The rate is 89%.
[0029] (2) Add intermediate (I) to a round bottom flask, add 10mL of anhydrous tetrahydrofuran as a solvent, place the round bottom flask under ice bath conditions, then add 15 mg of sodium hydride in batches, stir for a period of time and slowly drop Add 57 mg iodomethane. After reacting at room temperature for 12 hours, pass through a short column of silica gel, rinse the reactor and the sludge above the short column of gelatin with ethyl acetate, wash the filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com