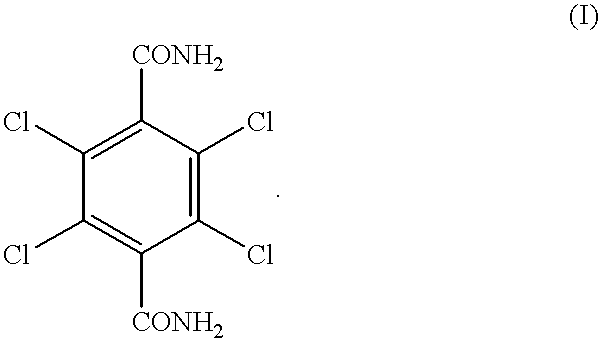
Process for producing 2, 3, 5, 6-tetrachloro-1, 4-benzenedicarboxylic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0045] Preparation of 2,3,5,6-tetrachloro-1,4-benzenedicarboxylic acid (compound 2) from 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide (compound 1)
[0046] 6.04 g (0.02 mol) of 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide and 17.65 g of sulfuric acid, consisting of 12.43 g of 96.3% sulfuric acid (containing 0.0256 mol of water) and 5.22 g of 26% fuming sulfuric acid (containing 0.017 mol of SO.sub.3), were charged into a reactor, and a hydrolysis of 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide was performed at 180.degree. C. for 6 hr under atmospheric pressure. The amount of water which was present prior to the reaction was 0.0086 mol because the SO.sub.3 in fuming sulfuric acid and the water in sulfuric acid were converted to sulfuric acid according to the formula (IV). After the completion of the reaction, precipitated crystals were collected by filtration, washed with water, and dried. As a result, 2,3,5,6-tetrachloro-1,4-benzenedicarboxylic acid was obtained.
[0047] Yield: 5.8 g (is...
example 2
[0050] Preparation of 2,3,5,6-tetrachloro-1,4-benzenedicarboxylic acid (compound 2) from 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide (compound 1)
[0051] 6.04 g (0.02 mol) of 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide and 17.64 g of sulfuric acid, consisting of 10.82 g of 96.3% sulfuric acid and 6.82 g of 26% fuming sulfuric acid, were charged into a reactor, and a hydrolysis of 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide was performed under atmospheric pressure. The temperature and reaction time were as specified in Table 1. After the completion of the reaction, the same processing as in Example 1 was conducted. As a result, 2,3,5,6-tetrachloro-1,4-benzenedicarboxylic acid was obtained.
[0052] Yield: 5.9 g (isolation yield 97%), and
[0053] IR (KBr): same as in Example 1.
example 3
[0054] Preparation of 2,3,5,6-tetrachloro-1,4-benzenedicarboxylic acid (compound 2) from 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide (compound 1)
[0055] 6.04 g (0.02 mol) of 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide and 17.65 g of sulfuric acid, consisting of 4.64 g of 96.3% sulfuric acid and 13.01 g of 26% fuming sulfuric acid, were charged into a reactor, and a hydrolysis of 2,3,5,6-tetrachloro-1,4-benzenedicarboxamide was performed under atmospheric pressure. The temperature and reaction time were as specified in Table 1. After the completion of the reaction, the same processing as in Example 1 was conducted. As a result, 2,3,5,6-tetrachloro-1,4-benzenedicarboxylic acid was obtained.
[0056] Yield: 4.1 g (isolation yield 68%), and
[0057] IR (KBr): same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com