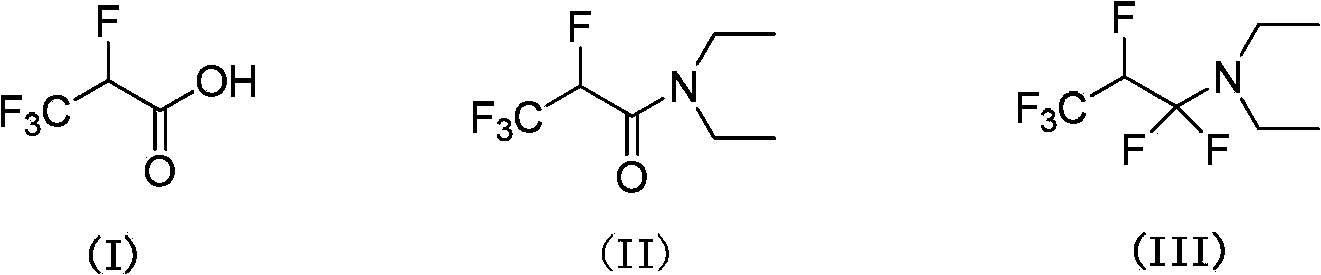
2,3,3,3-tetrafluoro propionic acid (i) synthesis method
A technique for the synthesis of tetrafluoropropionic acid, applied in organic chemistry, amide preparation, etc., can solve the problems of high cost of harmless treatment, environmental impact, etc., and achieve the effects of low production cost, mild reaction conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0044] Example 1: Preparation of 2,3,3,3-tetrafluoropropionic acid (I).
[0045]Put N,N-diethyl-2,3,3,3-tetrafluoropropionamide (II) (300g, 1.49mol), 37% hydrochloric acid (700ml), ethanol (1000ml), water (100ml) into the reaction In the bottle, heat up and reflux for 10 hours. After the reaction is complete, distill at atmospheric pressure and collect 120°C fractions to obtain 207g of I, with a yield of 95% and a purity of 98.5% (detected by gas chromatography);
[0046] The preparation conditions are:
[0047] N, N-dialkyl-2,3,3,3-tetrafluoropropionamide (II) is hydrolyzed under the action of hydrochloric acid.
[0048] The hydrolysis reaction uses a mixed solvent of ethanol and water with a volume ratio of 10:1.
[0049] The reaction temperature is controlled at 90-120°C.
[0050] The reaction time is controlled at 10-24 hours.
[0051] The molar ratio of N,N-dialkyl-2,3,3,3-tetrafluoropropionamide (II) to acid or base is 1:5-10.
[0052] The weight to volume ratio of ...
example 2
[0053] Example 2: Preparation of 2,3,3,3-tetrafluoropropionic acid (I).
[0054] Put N,N-diethyl-2,3,3,3-tetrafluoropropionamide (300g, 1.49mol), 98% sulfuric acid (600ml), ethanol (1000ml), water (100ml) into the reaction flask, Heated and refluxed for 12 hours, the reaction was completed, distilled at atmospheric pressure, and collected 120 ° C fractions to obtain 200 g of I, with a yield of 92% and a purity of 98.3% (detected by gas chromatography);
[0055] The preparation conditions are:
[0056] N, N-dialkyl-2,3,3,3-tetrafluoropropionamide (II) is hydrolyzed under the action of hydrochloric acid.
[0057] The hydrolysis reaction uses a mixed solvent of ethanol and water with a volume ratio of 10:1.
[0058] The reaction temperature is controlled at 90-120°C.
[0059] The reaction time is controlled at 10-24 hours.
[0060] The molar ratio of N,N-dialkyl-2,3,3,3-tetrafluoropropionamide (II) to acid or base is 1:5-10.
[0061] The weight to volume ratio of N,N-dialkyl...
example 3
[0062] Example 3: Preparation of 2,3,3,3-tetrafluoropropionic acid (I)
[0063] N, N-diethyl-2,3,3,3-tetrafluoropropionamide (II) (300g, 1.49mol), 98% sulfuric acid (600ml), ethanol (1000ml), water (100ml), dihydrate Cupric chloride (2.54g, 0.0149mol) was placed in a reaction flask, heated and refluxed for 5 hours, after the reaction was completed, distilled at atmospheric pressure, and 208g of I was obtained by collecting fractions at 120°C, with a yield of 96% and a purity of 99.0% (detected by gas chromatography) ;
[0064] The preparation conditions are:
[0065] N, N-dialkyl-2,3,3,3-tetrafluoropropionamide (II) is hydrolyzed under the action of hydrochloric acid.
[0066] The hydrolysis reaction uses a mixed solvent of ethanol and water with a volume ratio of 10:1.
[0067] The reaction temperature is controlled at 90-120°C.
[0068] The reaction time is controlled at 10-24 hours.
[0069] The molar ratio of N,N-dialkyl-2,3,3,3-tetrafluoropropionamide (II) to acid or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com