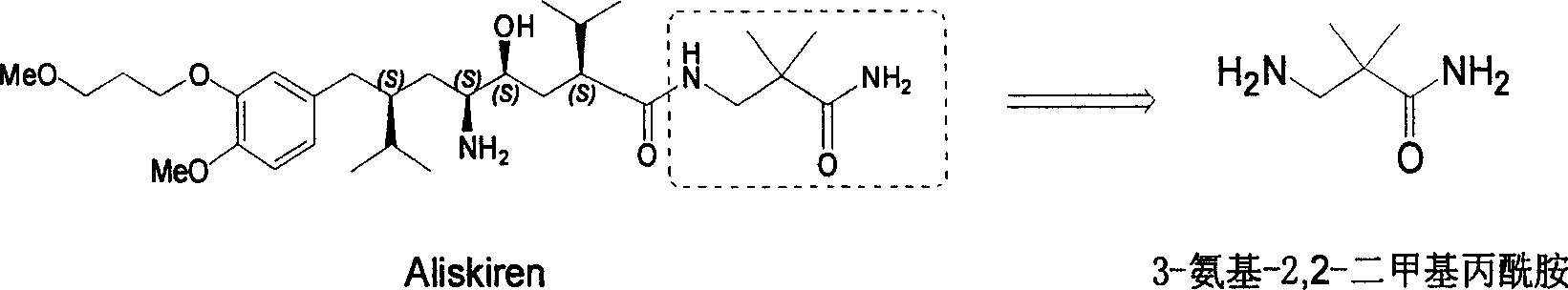
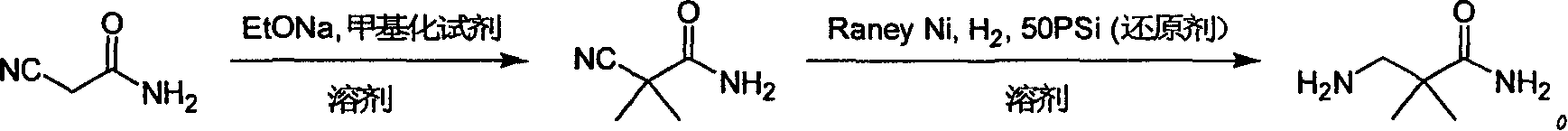Industrial preparation method for 3-amino-2, 2-dimethyl propionamide
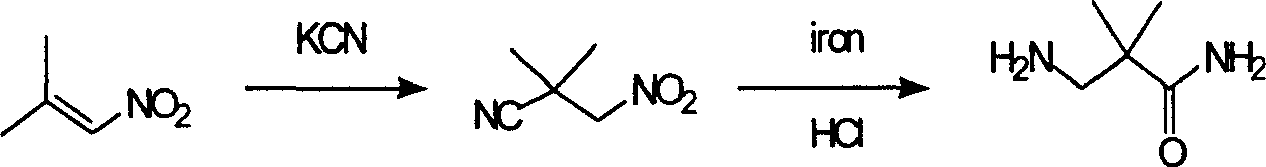
A technology of bismethylpropionamide and cyanoacetamide is applied in the field of industrialized preparation of 3-amino-2,2-bismethylpropionamide, which can solve the problems of rare raw materials and incapability of large-scale production, and achieves easy reaction Control, low preparation cost and reasonable selection of reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of 3-amino-2,2-dimethylpropanamide:
[0030] Step 1: Synthesis of Dimethylcyanoacetamide
[0031] Sodium ethoxide (204g, 3mol) was slowly added to 2000ml of absolute ethanol, stirred for half an hour after the addition, then added cyanoacetamide (84g, 1mol), at an internal temperature of 80°C, refluxed for 2 hours, and then cooled to At 30°C, methyl iodide (426 g, 3 mol) was added, the system was closed, and the reaction was carried out at room temperature for 12 hours. Evaporate the solvent to dryness, add 500ml of water, then extract six times with 250ml of dichloromethane, combine the dichloromethane layers, wash with saturated brine, dry over anhydrous sodium sulfate, spin dry, wash with 100ml of dichloromethane and filter to obtain the filter cake as Dimethylcyanoacetamide (90 g, 0.80 mol), 80% yield. 1 H NMR (400MHz, DMSO-d 6 ): δ7.63 (brs, 1H), 7.46 (brs, 1H), 1.58 (s, 6H); MS (m / z): 113 (M+H) + .
[0032] The second step: the synthesis of 3-amino-...
Embodiment 2
[0035] Synthesis of 3-amino-2,2-dimethylpropanamide:
[0036] Step 1: Synthesis of Dimethylcyanoacetamide
[0037] According to the process conditions and operation steps described in the first step of the above-mentioned Example 1, dimethylcyanoacetamide was prepared with a yield of 80%, and the test data were as shown in the first step of the above-mentioned Example 1.
[0038] The second step: the synthesis of 3-amino-2,2-bismethylpropionamide
[0039] Dissolve dimethylcyanoacetamide (5.6g, 0.05mol) in anhydrous methanol solution (40ml), add ammonia water (10ml) and Raney Ni catalyst (1.1g), and hydrogenate 3 Hour. The catalyst was removed by filtration and concentrated to obtain 3-amino-2,2-bismethylpropionamide (5.2 g, 0.045 mol) with a yield of 90%. Its test data is as shown in the second step of the above-mentioned embodiment 1.
Embodiment 3
[0041] Synthesis of 3-amino-2,2-dimethylpropanamide:
[0042] Step 1: Synthesis of Dimethylcyanoacetamide
[0043] According to the process conditions and operation steps described in the first step of the above-mentioned Example 1, dimethylcyanoacetamide was prepared with a yield of 80%, and the test data were as shown in the first step of the above-mentioned Example 1.
[0044] The second step: the synthesis of 3-amino-2,2-bismethylpropionamide
[0045] Dissolve dimethylcyanoacetamide (5.6g, 0.05mol) in anhydrous methanol solution (40ml), add 5% Pd / C catalyst (0.28g), and hydrogenate at 3 atmospheric pressure at 60°C for 3 hours . The catalyst was removed by filtration and concentrated to obtain 3-amino-2,2-bismethylpropionamide (5.0 g, 0.043 mol) with a yield of 86%. Its test data is as shown in the second step of the above-mentioned embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com