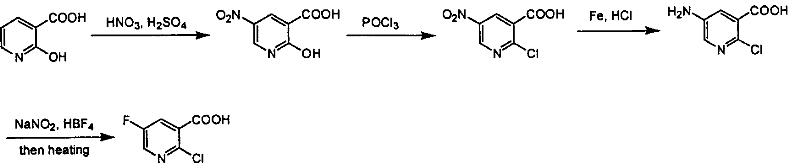
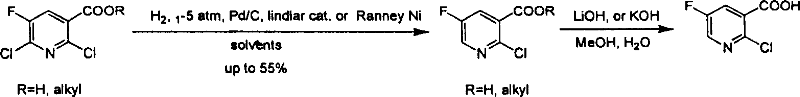
Industrialized method for preparing 2-chlorine-5-fluorin-nicotinic aicd
A technology of nicotinic acid and chlorinating agent, applied in the direction of organic chemistry, etc., can solve problems such as the need for chromatographic purification, and achieve the effect of reasonable selection of reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of 2-chloro-5-fluoro-nicotinic acid (POCl 3 , 50℃, 12h)
[0024] The first step: the synthesis of 5-fluoro-nicotinic acid
[0025] Add triethylamine (101.0g, 1.0mol) and 5% palladium carbon catalyst (4g) in 2,6-dichloro-5-fluoro-nicotinic acid (100g, 0.476mol) in absolute ethanol solution (2.4L) , hydrogenated at 3 atmospheres and room temperature for 12 hours. The catalyst was removed by filtration and concentrated to obtain the product (66.2 g, 0.468 mol), yield: 98%. 1 H NMR (400MHz, DMSO-d 6 ): 68.91(s, 1H), 8.81(d, J=2.8Hz, 1H), 8.09(dd, J=8.8&2.8Hz, 1H); Ms(M + +1, 142).
[0026] The second step: the synthesis of nitroxide-5-fluoro-nicotinic acid
[0027] 5-Fluoro-nicotinic acid (50g, 0.36mol) was dissolved in glacial acetic acid (500mL), and 30% H 2 o 2 (74mL, 0.72mol), heated to 100°C, reacted overnight, distilled off glacial acetic acid under reduced pressure, washed with water to obtain the product (53g, 0.34mol), yield: 95%. 1 H NMR (40...
Embodiment 2
[0031] Synthesis of 2-chloro-5-fluoro-nicotinic acid
[0032] In 2,6-dichloro-5-fluoro-nicotinic acid (50g, 0.238mol) in absolute ethanol solution (1.2L), add triethylamine (50.5g, 0.5mol) and Raney nickel catalyst (5g), Hydrogenation was carried out at 5 atmospheres and room temperature for 12 hours. The catalyst was removed by filtration and concentrated to obtain the product (32.8 g, 0.232 mol), yield: 97%.
[0033] In the process of preparing 2-chloro-5-fluoro-nicotinic acid, the corresponding products were prepared according to the nitrogen oxidation and chlorination process conditions and operating steps described in the second and third steps in the above example 1, and the test data were as above Example 1 shows.
Embodiment 3
[0035] Synthesis of 2-chloro-5-fluoro-nicotinic acid (POCl 3 +PCl 5 , 70℃, 12h)
[0036] According to the reaction conditions and operation methods described in the first step and the second step in the above example 1, the nitrogen oxide-5-fluoro-nicotinic acid intermediate was prepared. Then, nitroxide-5-fluoro-nicotinic acid (10 g, 63.7 mmol) was dissolved in POCl 3 (50mL), under ice bath, add PCl 5 , (20g) was heated to 50°C, stirred for 12h, and POCl was evaporated under reduced pressure 3 , adding 50 g of crushed ice, stirring, precipitated a beige solid, filtered to obtain 11 g of crude product, recrystallized from water to obtain pure product (6.7 g, 38.2 mmol), yield: 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com