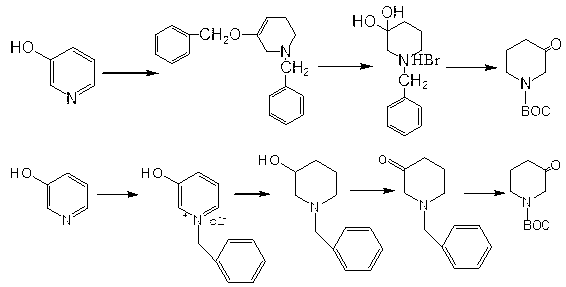
Method for synthesizing 1-BOC-3-piperidone
A 1-BOC-3-, piperidinone technology, applied in the field of medicine, can solve problems such as being unsuitable for industrial production, a large amount of waste water, waste residue, complicated operation process, etc., and achieve the effects of mild conditions, short synthesis steps and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The following examples are used to further illustrate the present invention, but it should not be interpreted as that the scope of the above-mentioned themes of the present invention is limited to the following examples, and all technologies realized based on the above-mentioned content of the present invention all belong to the scope of the present invention.
[0023] 1. Synthesis of Step 1 compound 2 (3-hydroxypiperidine):
[0024] 1. Preparation of sodium borohydride solution: 113.5g of sodium borohydride, 150ml of water and 40ml of 20% NaOH aqueous solution were mixed.
[0025] 2. Synthesis: Put 95.1g of compound 1 (3-hydroxypyridine) into a 1L reaction flask, add 300ml of NaOH aqueous solution with a mass percentage of 20%, raise the temperature to 60°C, add sodium borohydride solution dropwise, keep warm for 1h, drop After the addition, the temperature was raised to 80°C and kept for 5 hours. After the heat preservation was completed, stirred and cooled to room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com