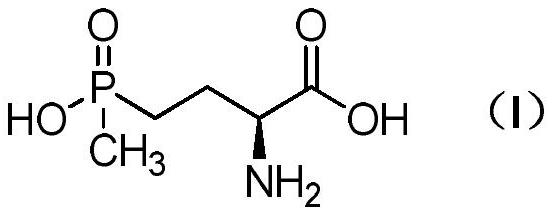
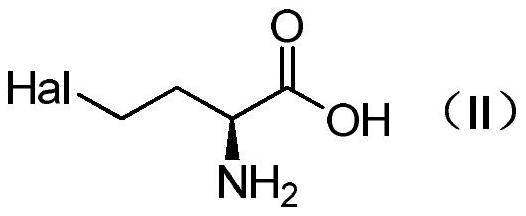
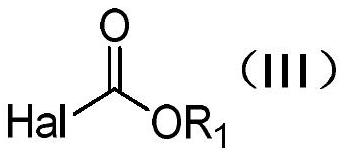
Method for preparing L-glufosinate-ammonium
A technology for glufosinate-ammonium and a compound is applied in the field of preparing L-glufosinate-ammonium, which can solve the problems of difficulty in realizing high-efficiency industrial production, expensive chiral resolution reagents, low industrial application value and the like, and achieves low price, short synthesis steps, The effect of less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Synthesis of compound 2
[0049]
[0050] Dissolve 50 g of L-homoserine (1) in 36% HCl solution (120 g), add 0.5 g of tetrabutylammonium bromide, react in a closed pressure-resistant reactor at 80° C. for 9 h, and cool naturally to room temperature. The reaction solution was detected by LC, and the reaction of the raw materials was complete. The reaction solution was directly suction-filtered and dried to obtain 50 g of light yellow solid compound 2 with a yield of 87%.
[0051] (2) Synthesis of compound 3
[0052]
[0053] Under a nitrogen atmosphere, 50 g of compound 2 (0.364 mol) and 150 mL of toluene were added to a 500 mL three-necked flask, and the temperature was raised to 40 ° C. At this temperature, 55 g (0.185 mol) of triphosgene in toluene (50 mL) was added dropwise. After the reaction was complete, the solvent was distilled off under reduced pressure, and then recrystallized from ethyl acetate to obtain compound 3 as a light yellow solid with a yi...
Embodiment 2
[0061]According to the method of Example 1, the reaction conditions of step (2) were changed, and the results are shown in Table 1 below. Wherein, the amount of triphosgene used is 0.5 equivalents, the amount of phosgene used is 1.5 equivalents, the amount of diphosgene used is 0.75 equivalents, and the amount of ethyl chloroformate is 1.5 equivalents.
[0062] Table 1
[0063] serial number Reagent solvent temperature reflex Compound 3 Yield 1 Triphosgene toluene 60℃ 61% 2 Triphosgene toluene 110℃ 51% 3 Triphosgene Tetrahydrofuran 40℃ 53% 4 Triphosgene Tetrahydrofuran 60℃ 73% 5 Triphosgene Dichloromethane 40℃ 51% 6 Triphosgene 1,4-dioxane 40℃ 45% 7 Triphosgene 1,4-dioxane 90℃ 48% 8 Triphosgene MTBE 60℃ 59% 9 Triphosgene 1,2-Dichloroethane 40℃ 41% 10 Triphosgene ethyl acetate 40℃ 44% 11 Triphosgene ethyl acetate 60℃ 62% 12 Phosgene Tetrahydrofur...
Embodiment 3
[0066] (1) Synthesis of compound 2'
[0067]
[0068] Dissolve 50 g of L-homoserine (1) in 48% HBr in CH 3 In COOH solution (120g), react in a closed pressure-resistant reactor at 80°C for 6h, and after naturally cooling to room temperature, the reaction solution is detected by LC and LC-MS, and the raw materials are completely reacted. The reaction solution is directly suction filtered and dried to obtain 72g of brown yellow solid compound 2', yield 95%.
[0069] (2) Synthesis of compound 3'
[0070]
[0071] Under a nitrogen atmosphere, 50g of compound 2' and 150mL of tetrahydrofuran were added to a 500mL three-necked flask, and the temperature was raised to 60°C. At this temperature, 122g of triphosgene in THF (100mL) was added dropwise. After the solvent was distilled off under pressure, it was recrystallized from ethyl acetate to obtain 36 g of a light yellow solid with a yield of 73%.
[0072] (6) Synthesis of compound 4
[0073]
[0074] Under a nitrogen at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com