High-birefringence liquid crystal compound, and preparation method and composition thereof
A liquid crystal compound and liquid crystal composition technology, applied in chemical instruments and methods, liquid crystal materials, etc., can solve problems such as crystallization, and achieve the effects of low raw material cost, low melting enthalpy, and short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
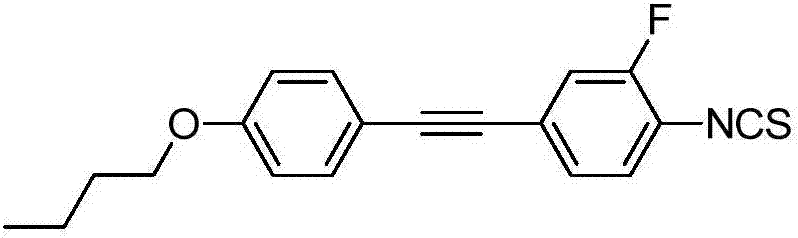
[0047] Synthesis of 4-((4-propenyloxy)phenyl)ethynyl)-2-fluoro-1-isothiocyanatobenzene:
[0048] The specific structure is as follows:
[0049]
[0050] The preparation process is as follows:
[0051] Step 1: Synthesis of 1-(propenyloxy)-4-iodobenzene
[0052]
[0053] Under nitrogen protection, p-iodophenol (176.08g, 0.8mol), 3-bromo-1-propene (145.2g, 1.2mol), K 2 CO 3 (221g, 1.6mol), ethanol 800mL. Turn on heating and stirring, stop stirring after reflux reaction for 2 hours, and naturally cool down to room temperature for aftertreatment. Filter out the insoluble matter, spin evaporate ethanol, add n-heptane to dissolve the organic matter, wash with water until neutral, dry over anhydrous magnesium sulfate, filter, spin the filtrate to dryness, and go through column chromatography, elute with n-heptane, eluent After the solvent was removed by rotary evaporation, 201 g of colorless liquid was obtained, which was the intermediate 1-(propenyloxy)-4-iodobenzene. GC ...
Embodiment 2
[0073] Synthesis of 4-((4-(2,2-difluorovinyl)phenyl)ethynyl)-2-fluoro-isothiocyanatobenzene:
[0074]
[0075] Using 4-iodoaniline instead of 2-fluoro-4-iodoaniline in step (4) of Example 1, the same method as in Example 1 was used to synthesize 1-(propenyloxy)-4-((4-isosulfur cyanophenyl)ethynyl)benzene.
[0076] Structure Identification:
[0077] 1 H-NMR (δ, CDCl 3 ):4.552-4.568(m,2H),5.296-5.320(m,1H),5.434-5.443(m,1H),6.013-6.090(m,1H),6.879-6.908(m,2H),7.170-7.192 (m,2H),7.433-7.482(m,4H); MS(70eV)m / z(%):290.9(M + ,63), 221.9.0(19), 189.9(8), 162.9(17), 149.9(100).
[0078] The above structural identification data show that the synthesized compound is indeed 1-(propenyloxy)-4-((4-isothiocyanatophenyl)ethynyl)benzene.
[0079] Use DSC to test the liquid crystal phase transition temperature of 1-(propenyloxy)-4-((4-isothiocyanatophenyl)ethynyl)benzene at a temperature of 3°C / min. The result is: Cr 104.8I, melting The enthalpy value is 21.35 kJ / mol. The monomeric ...
Embodiment 3
[0084] Synthesis of 1-((4-(propenyloxy)phenyl)ethynyl)-2-fluoro-4-isothiocyanatobenzene:
[0085]
[0086] Using 3-fluoro-4-iodoaniline instead of 2-fluoro-4-iodoaniline in step (4) of Example 1, the same method as in Example 1 is used to synthesize 1-((4-(propenyloxy)benzene (yl)ethynyl)-2-fluoro-4-isothiocyanatobenzene.
[0087] Structure Identification:
[0088] 1 H-NMR (δ, CDCl 3 ): 4.555-4.571(m,2H),5.297-5.321(q,1H),5.403-5.440(q,1H),6.012-6.089(m,1H),6.884-6.912(m,2H),6.953-7.007 (m,2H),7.437-7.487(m,3H); MS(70eV)m / z(%):308.9(M + ,66),267.9(100),239.9(22),207.9(8),182(16),156(2).
[0089] The above structural identification data show that the synthesized compound is indeed 1-((4-(propenyloxy)phenyl)ethynyl)-2-fluoro-4-isothiocyanatobenzene.
[0090] Use DSC to test the liquid crystal phase transition temperature of 1-((4-(propenyloxy)phenyl)ethynyl)-2-fluoro-4-isothiocyanatobenzene at a temperature of 3°C / min. The result is: Cr 66.35 N 81.35 I, the melting poi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com