Application of n-butyllithium in catalyzing cyanosilylation reaction of aldehyde and silane
A technology of n-butyllithium and silyl cyanosilane is applied in the application field of organolithium compounds, which can solve the problems such as the limited scope of substrate trial and the difficulty of catalyst synthesis, and achieve the effects of simple and controllable reaction process, easy acquisition and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment one: n-BuLi catalyzes the reaction of benzaldehyde and TMSCN
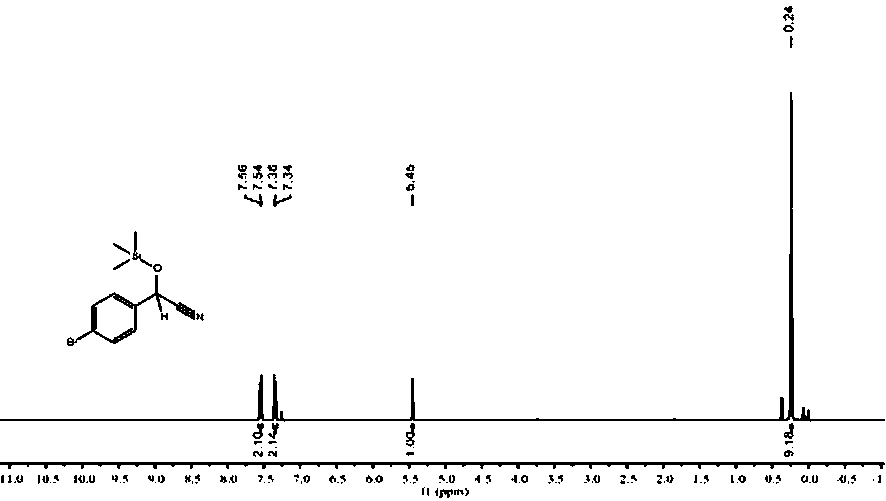
[0029] Under an inert gas atmosphere, add benzaldehyde (101.6 μL, 1 mmol), trimethylsilyl cyanide (137.6 μL, 1.1 mmol) and finally add 0.1% catalyst n-BuLi (20 μL, 0.01 M in THF), react at rt for 30 min, pipette a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 7.41 – 7.30 (m, 5H, ArH), 5.42 (s, 1H, CH), 0.16 (s, 9H, CH 3 ), figure 1 its NMR spectrum.
Embodiment 2
[0033] Embodiment two: n-BuLi catalyzes the reaction of benzaldehyde and TMSCN
[0034] Under an inert gas atmosphere, add benzaldehyde (101.6 μL, 1 mmol), trimethylsilyl cyanide (150.1 μL, 1.2 mmol) and finally add 0.1 % catalyst to the dehydrated and deoxygenated reaction flask with a pipette gun n-BuLi (20 μL, 0.01 M in THF), react at rt for 30 min, pipette a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. The NMR data of the product are the same as in Example 1.
[0035] Comparative example:
[0036]Under an inert gas atmosphere, add benzaldehyde (101.6 μL, 1 mmol) and trimethylsilyl cyanide (150.1 μL, 1.2 mmol) sequentially to the dehydrated and deoxygenated reaction vial with a pipette gun, and react at rt for 30 min After that, use a dropper to draw a drop into the nuclear magnetic tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 11%.
Embodiment 3
[0037] Embodiment three: n-Butyllithium catalyzes the reaction of benzaldehyde and TMSCN
[0038] Under an inert gas atmosphere, add benzaldehyde (101.6 μL, 1 mmol), trimethylsilyl cyanide (125.1 μL, 1 mmol) and finally add 0.1% catalyst to the reaction vial after dehydration and deoxygenation n-BuLi (20 μL, 0.01 M in THF), react at rt for 30 min, pipette a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 93%. The NMR data of the product are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com