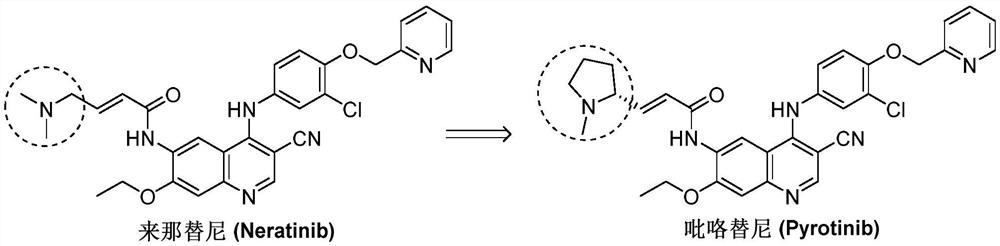
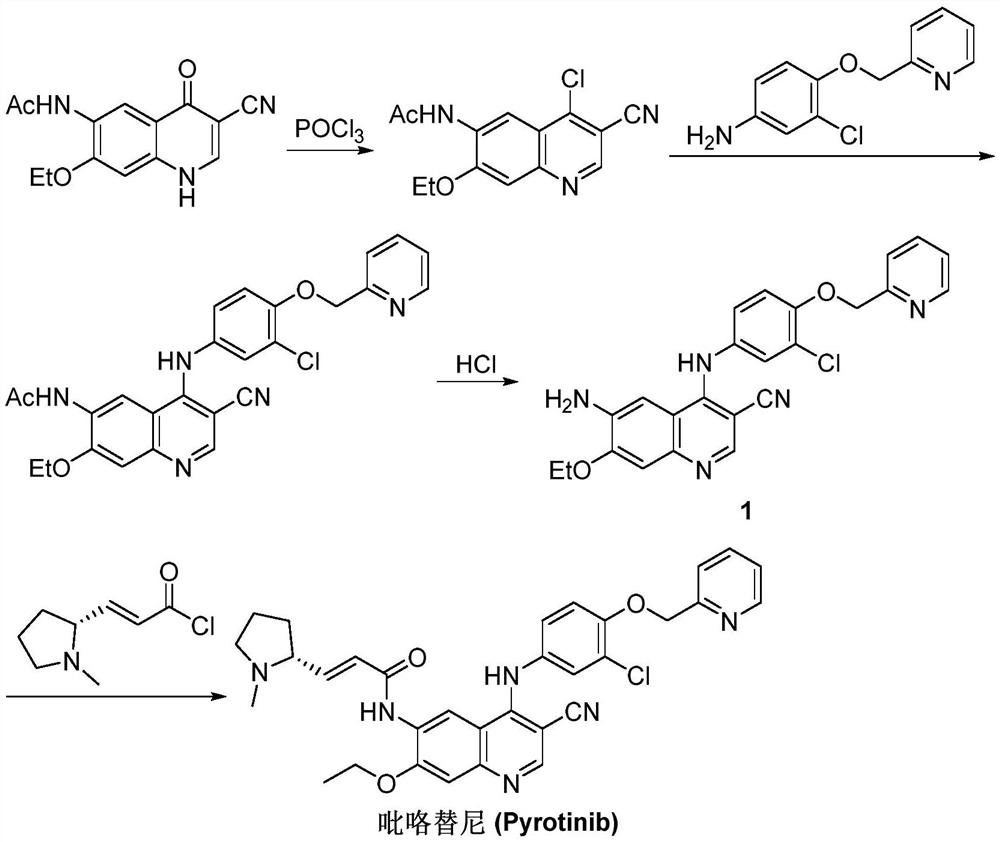
Synthesis method of pyrotinib
A technology of pyrotinib and synthetic method, applied in the field of pharmaceutical chemical synthesis, can solve the problems of high cost, easy oxidation and deterioration, low industrial production value and significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (A) Preparation of [3-chloro-4-(pyridin-2-ylmethoxy)phenyl]boronic acid:
[0030] Dissolve 2-[(4-bromo-2-chlorophenoxy)methyl]pyridine (5.0g, 17mmol) as formula I in tetrahydrofuran (80mL), cool to -78°C, and slowly add 1.6M n- Butyl lithium in n-hexane solution (12mL), the reaction mixture was stirred at -78°C for 2h, then trimethyl borate (2.5g, 24mmol) was slowly added, the reaction mixture was stirred at -78°C for 1h, then naturally raised to 20°C and stirred for 10h , slowly add methanol (50mL), add dropwise dilute hydrochloric acid to adjust pH = 3, cool and crystallize for 3h, filter with suction, and dry in vacuo to obtain [3-chloro-4-(pyridin-2-ylmethoxy) as formula II Phenyl] boronic acid (4.0g), yield 91%, the reaction formula of this step is:
[0031]
[0032] (B) preparation of pyrotinib:
[0033] (2E)-N-(4-amino-3-cyano-7-ethoxyquinolin-6-yl)-3-[(2R)-1-methylpyrrolidin-2- Base] acrylamide (5.0g, 14mmol) and [3-chloro-4-(pyridin-2-ylmethoxy)phenyl]bor...
Embodiment 2
[0036] (A) Preparation of [3-chloro-4-(pyridin-2-ylmethoxy)phenyl]boronic acid:
[0037]First, 2-[(4-bromo-2-chlorophenoxy)methyl]pyridine (12.5g, 42mmol) was dissolved in tetrahydrofuran (200mL), cooled to -78°C, and slowly added dropwise with 1.6M n-butyllithium n-Hexane solution (33mL,), the reaction mixture was stirred at -78°C for 2h, then slowly added triethyl borate (10.0g, 69mmol), the reaction mixture was stirred at -78°C for 1h, naturally raised to 25°C and stirred for 8h, slowly added Methanol (60mL), dilute hydrochloric acid was added dropwise to adjust pH=3, cooled and crystallized for 3h, suction filtered, and vacuum dried to obtain [3-chloro-4-(pyridin-2-ylmethoxy)phenyl]boronic acid (10.0g ), yield 91%;
[0038] (B) preparation of pyrotinib:
[0039] (2E)-N-(4-amino-3-cyano-7-ethoxyquinolin-6-yl)-3-[(2R)-1-methylpyrrolidin-2-yl]acrylamide ( 11.0g, 30mmol), [3-chloro-4-(pyridin-2-ylmethoxy)phenyl]boronic acid (10.0g, 38mmol), copper acetate (2.0g, 10mmol) and...
Embodiment 3
[0041] (A) Preparation of [3-chloro-4-(pyridin-2-ylmethoxy)phenyl]boronic acid:
[0042] Dissolve 2-[(4-bromo-2-chlorophenoxy)methyl]pyridine (30.0g, 0.10mol) in tetrahydrofuran (400mL), cool to -78°C, and slowly add 1.6M n-butyl lithium dropwise n-hexane solution (90mL), the reaction mixture was stirred at -78°C for 2h, then triisopropyl borate (35.0g, 0.19mol) was slowly added, the reaction mixture was stirred at -78°C for 1h, then naturally raised to 25°C and stirred for 6h, Add methanol (80mL) slowly, add dilute hydrochloric acid dropwise to adjust pH=3, cool and crystallize for 3h, filter with suction, and dry in vacuo to obtain [3-chloro-4-(pyridin-2-ylmethoxy)phenyl]boronic acid ( 24.0g), yield 91%;
[0043] (B) preparation of pyrotinib:
[0044] (2E)-N-(4-amino-3-cyano-7-ethoxyquinolin-6-yl)-3-[(2R)-1-methylpyrrolidin-2-yl]acrylamide ( 24.0 g, 66 mmol), [3-chloro-4-(pyridin-2-ylmethoxy)phenyl]boronic acid (24.0 g, 91 mmol), copper sulfate (5.0 g, 31 mmol) and pyridi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com