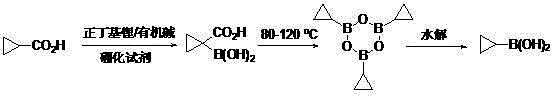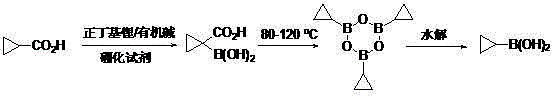Method for preparing cyclopropyl boronic acid
A technology of cyclopropylboronic acid and cyclopropylcarboxylic acid, which is applied in the field of fine chemical intermediate synthesis, can solve the problems of safety, many coupling by-products, and poor stability of boronizing reagents in the environment or experimental personnel, and avoids high toxicity. The use of mercury oxide and the effect of simplifying the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of 1-carboxycyclopropylboronic acid:
[0023] Under the protection of nitrogen, add 120 ml of anhydrous 2-methyltetrahydrofuran and diisopropylamine (0.24 mol) into the reaction flask equipped with a dropping device, cool to -70°C, and start adding 2.5M n-butyllithium solution dropwise 88 ml. After completion of the dropwise addition, keep stirring for 1 hour. Dissolve cyclopropyl formic acid (0.1 mol) in 50 ml of anhydrous tetrahydrofuran, mix well, transfer to the above-mentioned dropping funnel, start adding the mixed solution dropwise, and keep the reaction temperature at -70°C to - 60°C. After the dropwise addition, continue to stir and react for 1-2 hours, and add deuterated water to confirm that the conversion rate is greater than 95%. Then, a mixed solution of trimethyl borate (0.20 mol) dissolved in 40 ml of anhydrous 2-methyltetrahydrofuran was added into the dropping funnel, and the reaction temperature was kept at -70°C to -60°C during the dr...
Embodiment 2
[0029] Synthesis of 1-carboxycyclopropylboronic acid:
[0030] Under the protection of nitrogen, add 120 ml of anhydrous tetrahydrofuran and 2,2,6,6-tetramethylpiperidine (0.26 mol) into the reaction flask equipped with a dropping device, cool to -50 °C, and start dropping 2.5 M n-BuLi solution 90 ml. After completion of the dropwise addition, keep stirring for 1 hour. Dissolve cyclopropyl formic acid (0.1 mol) in 50 ml of anhydrous tetrahydrofuran, mix well, transfer to the above-mentioned dropping funnel, start adding the mixed solution dropwise, and keep the reaction temperature at -70°C to - 60°C. After the dropwise addition, continue to stir and react for 1-2 hours, and add deuterated water to confirm that the conversion rate is greater than 95%. Then, a mixed solution of triisopropyl borate (0.30 mol) dissolved in 40 ml of anhydrous tetrahydrofuran was added into the dropping funnel, and the reaction temperature was kept at -70°C to -60°C during the dropwise additi...
Embodiment 3
[0036] Synthesis of 1-carboxycyclopropylboronic acid:
[0037] Under the protection of nitrogen, add 120 ml of anhydrous tetrahydrofuran and hexamethyldisilazane (0.26 mol) into the reaction flask equipped with a dropping device, cool to -40°C, and start to add 2.5M n-butyllithium solution dropwise 96 ml. After completion of the dropwise addition, keep stirring for 1 hour. Dissolve cyclopropyl formic acid (0.11 mol) in 55 ml of anhydrous tetrahydrofuran, mix well and transfer it into the above-mentioned dropping funnel, start adding the mixed solution dropwise, during the dropping process, keep the reaction temperature at -70°C to - 60°C. After the dropwise addition, continue to stir and react for 1-2 hours, and add deuterated water to confirm that the conversion rate is greater than 95%. Then, a mixed solution of tris(trimethylsilyl)boron (0.20 mol) dissolved in 40 ml of anhydrous 2-methyltetrahydrofuran was added to the dropping funnel, and the reaction temperature was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com