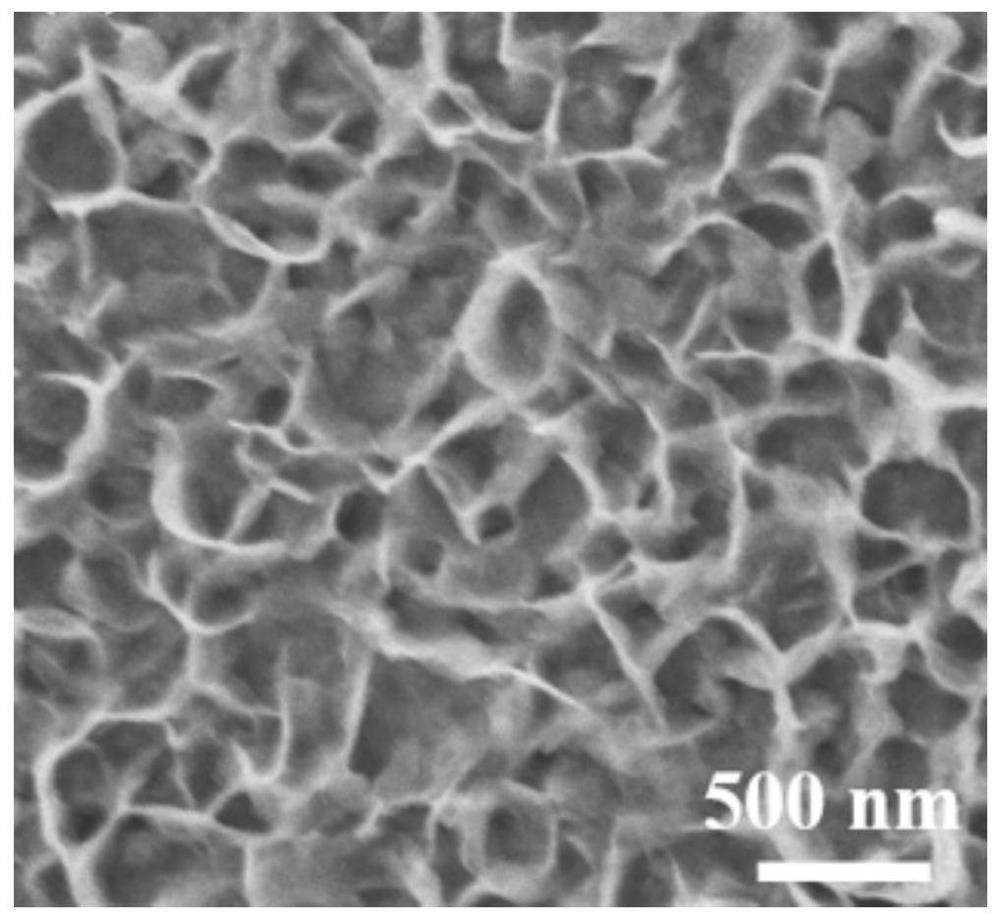
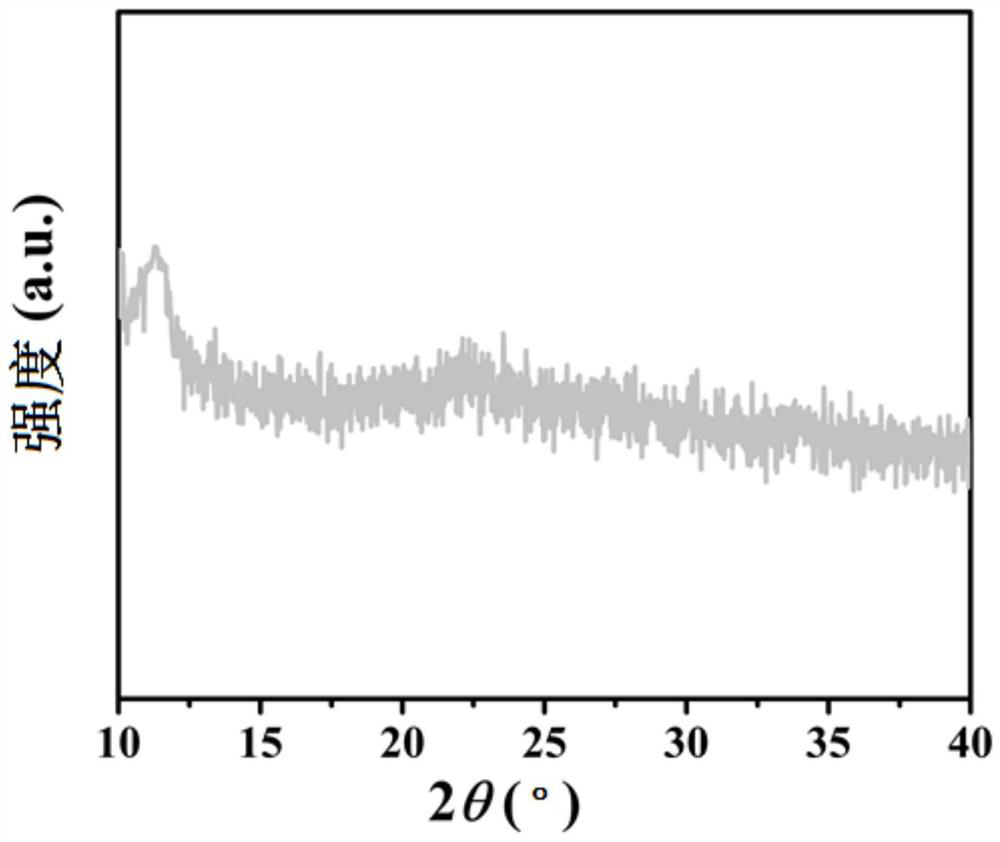
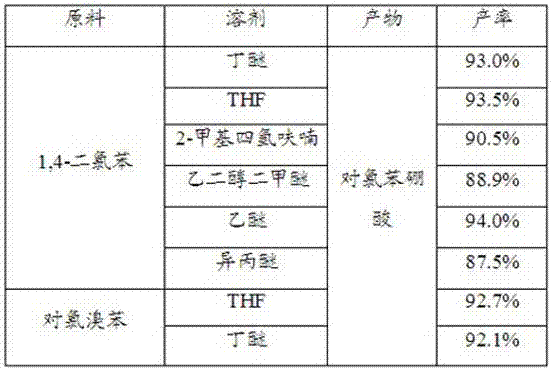
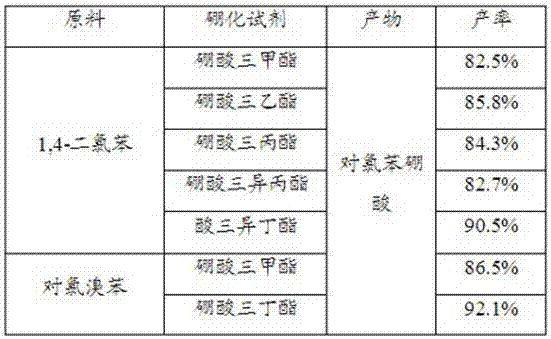
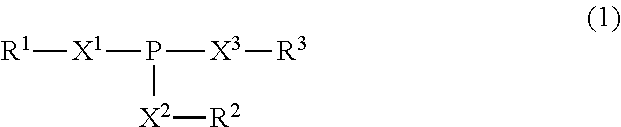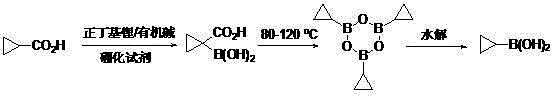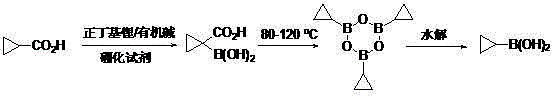Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
161 results about "Borylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen bond activation. Metal-catalyzed C–H borylation reactions utilize transition metals to directly convert a C–H bond into a C–B bond. This route can be advantageous compared to traditional borylation reactions by making use of cheap and abundant hydrocarbon starting material, limiting prefunctionalized organic compounds, reducing toxic byproducts, and streamlining the synthesis of biologically important molecules. Boronic acids, and boronic esters are common boryl groups incorporated into organic molecules through borylation reactions. Boronic acids are trivalent boron-containing organic compounds that possess one alkyl substituent and two hydroxyl groups. Similarly, boronic esters possess one alkyl substituent and two ester groups. Boronic acids and esters are classified depending on the type of carbon group (R) directly bonded to boron, for example alkyl-, alkenyl-, alkynyl-, and aryl-boronic esters. The most common type of starting materials that incorporate boronic esters into organic compounds for transition metal catalyzed borylation reactions have the general formula (RO)₂B-B(OR)₂. For example, Bis(pinacolato)diboron (B₂Pin₂), and bis(catecholato)diborane (B₂Cat₂) are common boron sources of this general formula.
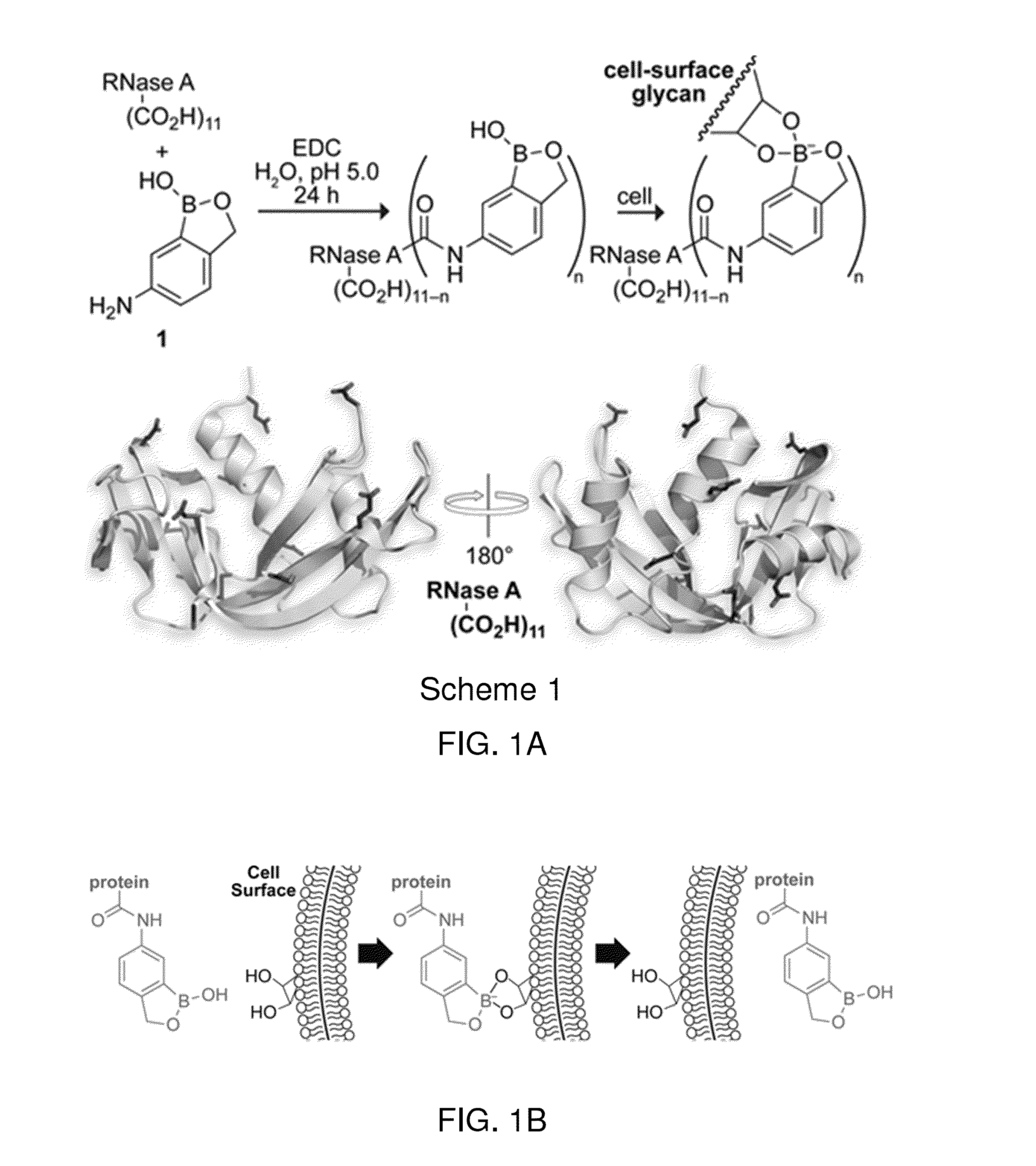
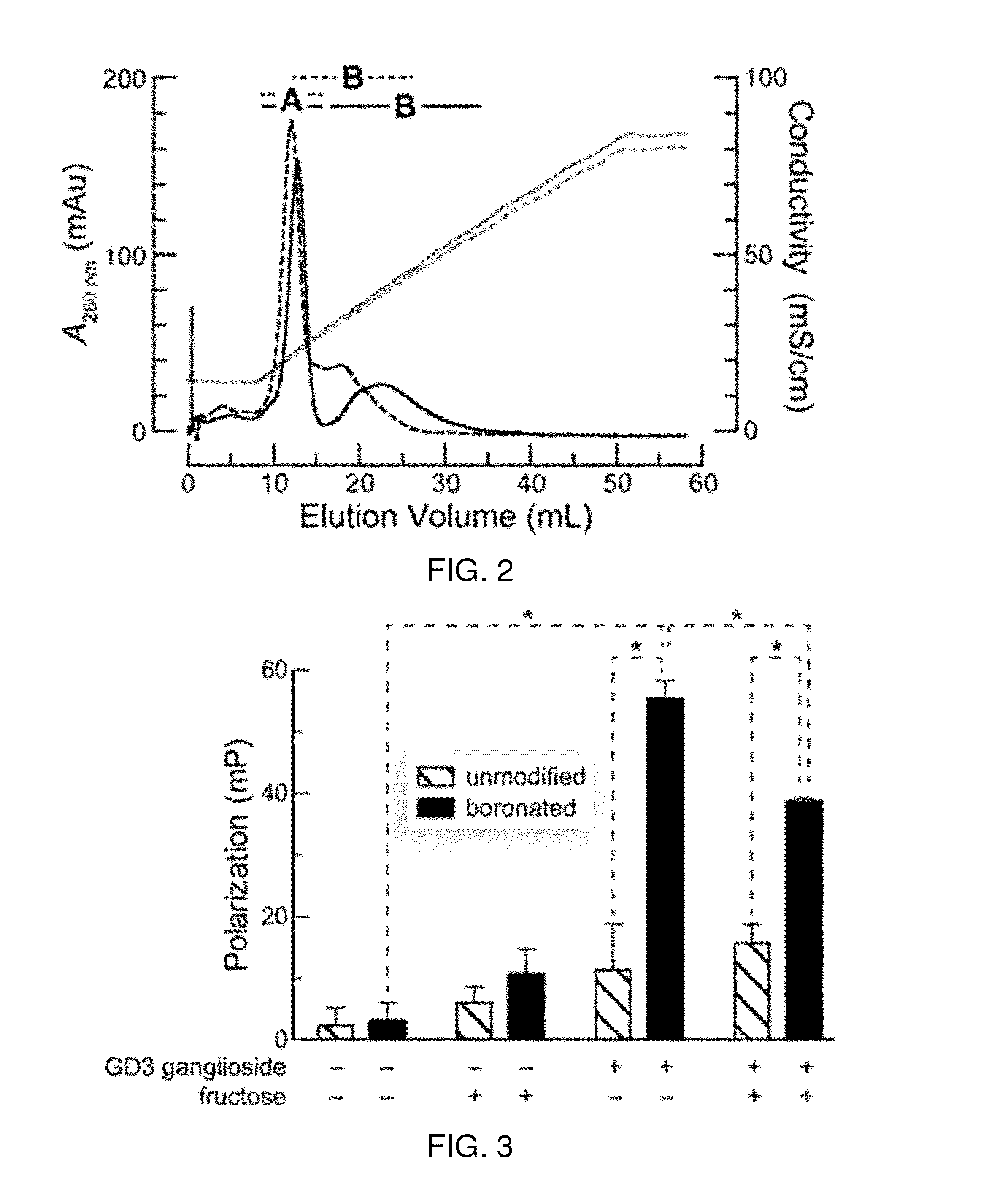
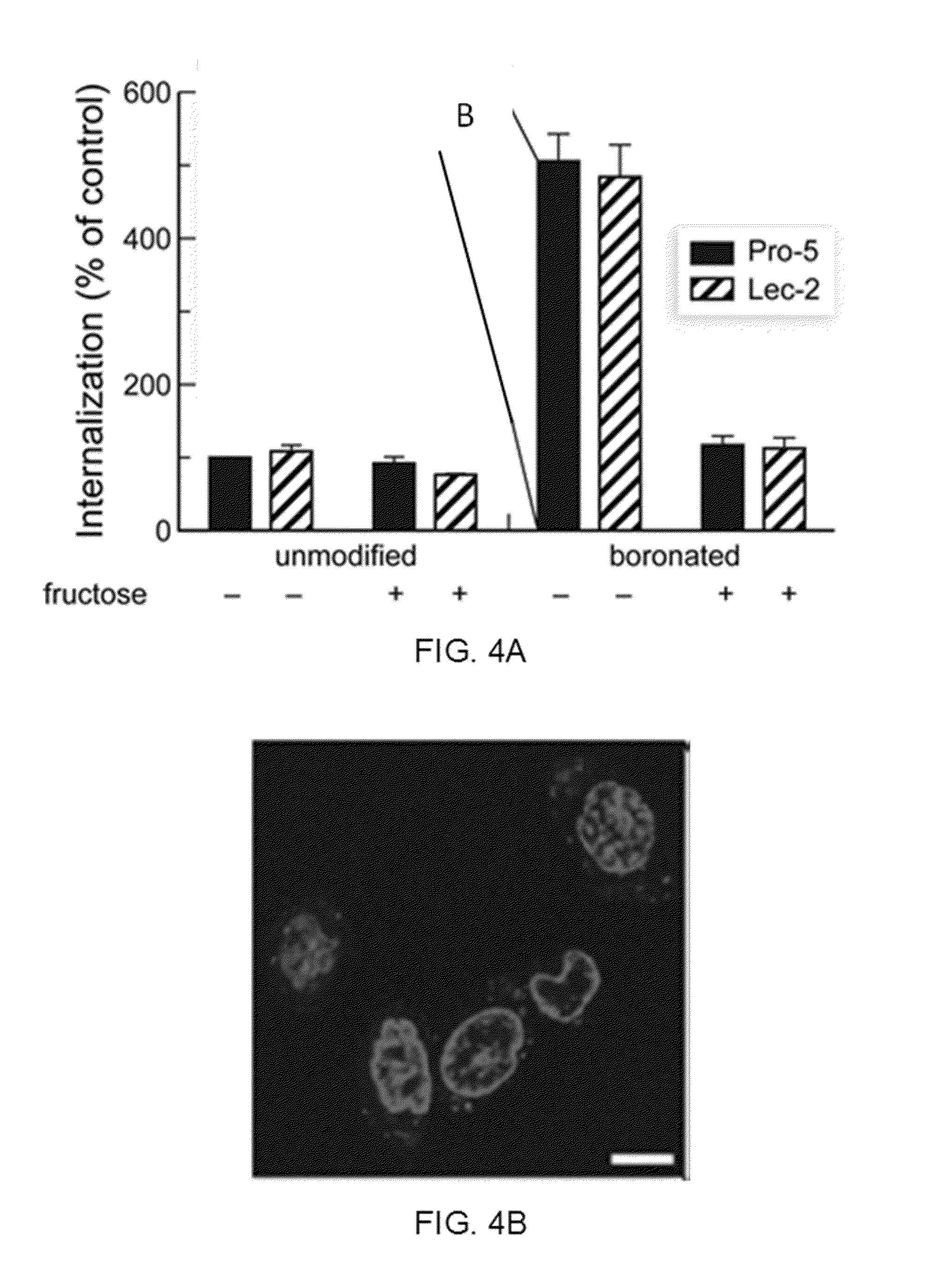
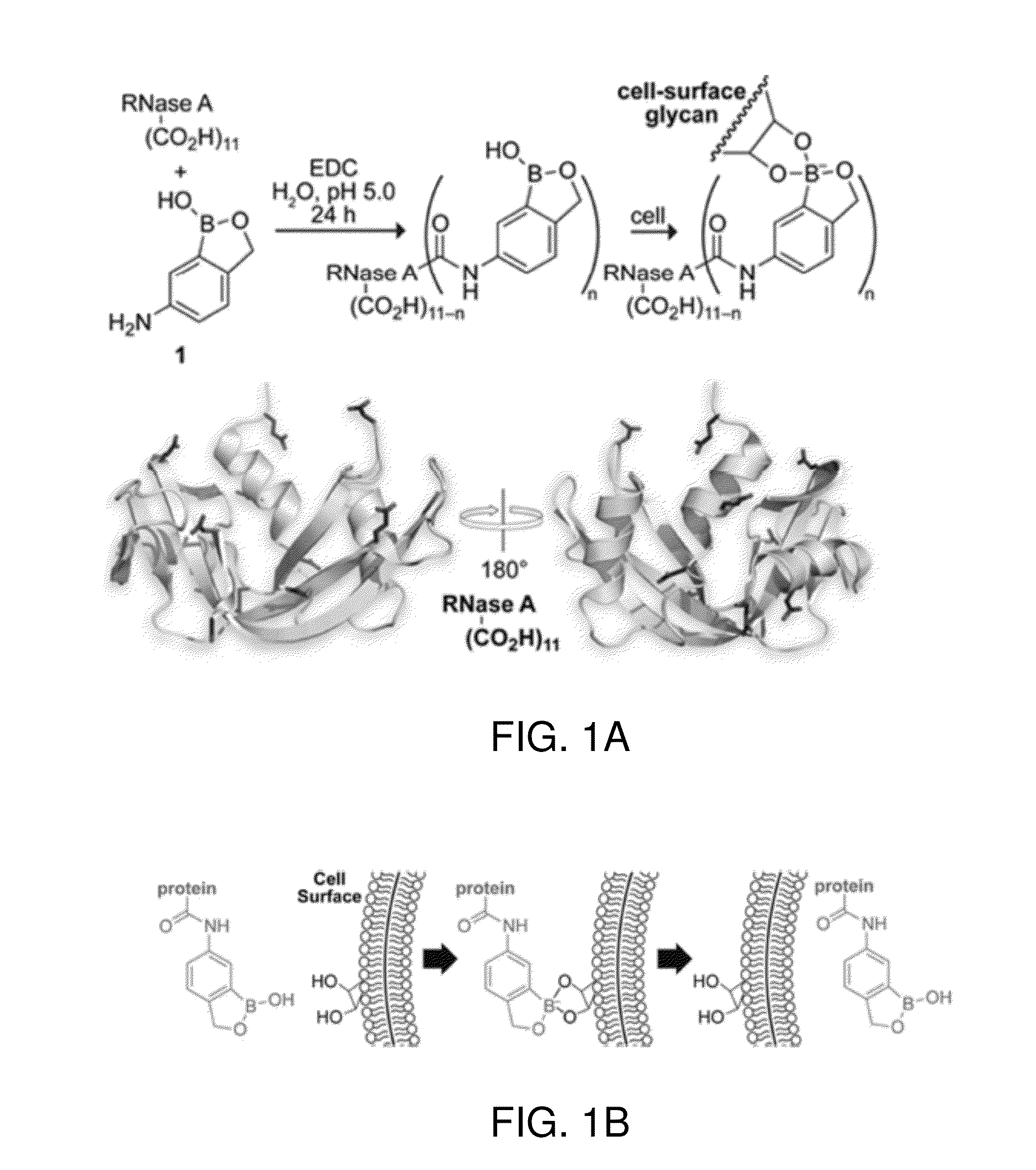
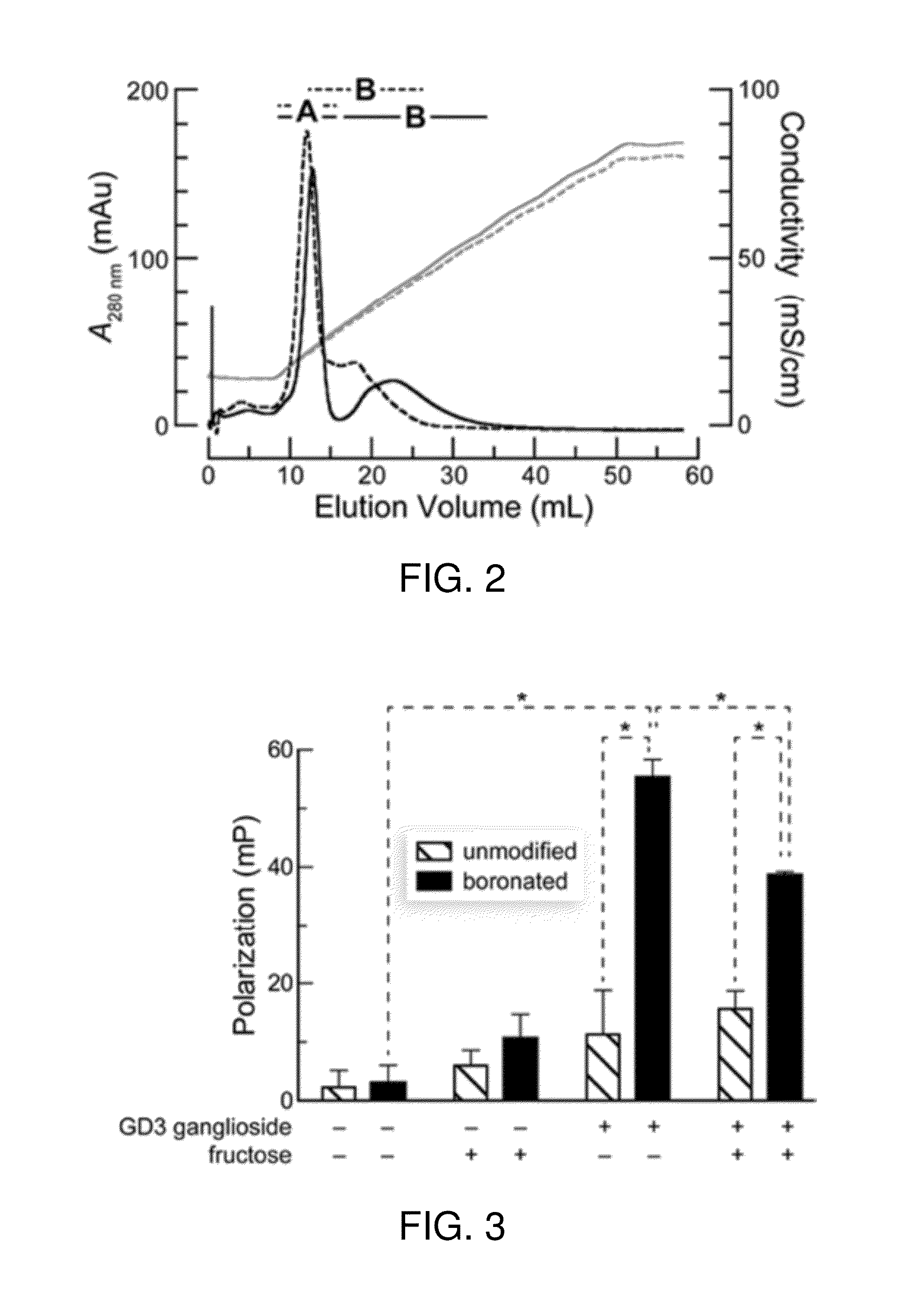
Boronate-Mediated Delivery of Molecules into Cells
Methods for enhancing cellular uptake of cargo molecules by boronating the cargo molecule, particularly with one or more phenylboronic acid groups. Cellular uptake includes at least partial uptake into the cytosol. Boronation includes ligating, crosslinking or otherwise bonding one or more phenylboronic acids substituted to contain a reactive group to a cargo molecule. Boronation also includes ligating, crosslinking or otherwise bonding a phenylboronated oligopeptide to a cargo molecule. The phenylboronate groups are optionally conjugated to the cargo molecule via linking moieties that can be selectively cleaved, such cleavable linkers can allow the phenylboronate groups to be removed from the cargo molecule after the boronated cargo molecule is introduced into the cell. The invention includes certain phenylboronates which are boronation reagents, certain boronated oligopeptides and certain boronated peptides and proteins. The invention also includes kits for enhancing cellular uptake of cargo molecules by boronation with one or more phenylboronates or boronated oligopeptides.
Owner:WISCONSIN ALUMNI RES FOUND
C5/C6 alkane isomerization catalyst loaded with nickel boride as well as preparation method and application method thereof
The invention relates to a C5 / C6 alkane isomerization catalyst loaded with nickel boride as well as a preparation method and an application method thereof. The catalyst comprises a carrier and the nickel boride the weight of which is 1-5% of that of the carrier, wherein the carrier is formed by aluminium oxide and an H beta molecular sieve; the weight ratio of the aluminium oxide to the H beta molecular sieve is (1:9)-(9:1). According to the catalyst provided by the invention, halogen components are not needed to be added, and noble metals are not used, so that the environmental pollution is avoided and the catalyst cost is reduced; besides, the catalyst can be directly used for isomerization process without requiring pre-hydrogenation for reduction. Tests prove that when the catalyst is used for isomerization reaction for catalyzing n-hexane and n-pentane, the activity of the catalyst is higher, and the isoalkane selectivity and the catalyst stability are better.
Owner:BC P INC CHINA NAT PETROLEUM CORP
Bioreversible boronates for delivery of molecules into cells
ActiveUS20160024122A1Immobilised enzymesSilicon organic compoundsPhenylboronic acidOrganic chemistry
Methods for enhancing cellular uptake of cargo molecules by boronating the cargo molecule, particularly with one or more phenylboronic acid groups. Boronation reagents for reversible boronation of cargo molecules, particularly, cargo molecules having one or more amino groups are provided.
Owner:WISCONSIN ALUMNI RES FOUND
Incorporation of functional groups into polymers using C-H activation
Designed functionality is incorporated onto a preformed aromatic polymer. The preformed aromatic polymer is provided in a reactive medium. Within that reactive medium is provided a borylation reagent and a catalyst for C—H borylation. A; and a C—H position on an aromatic ring on the preformed aromatic polymer is catalytically borylated with the borylating agent to form a borylated aromatic moiety on the preformed aromatic polymer as an incorporated boryl functionality. That boryl functionality may then be reacted with designed alternative functionalities.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA LAS VEGAS THE
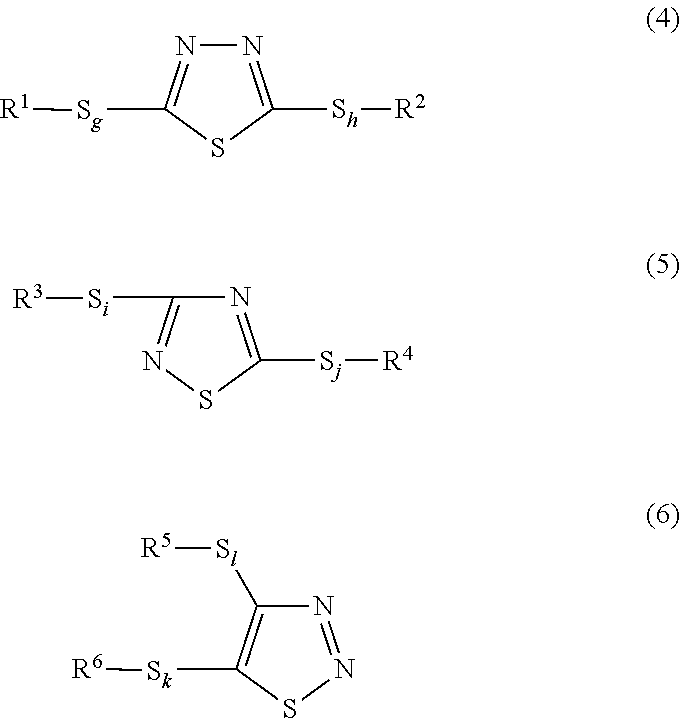
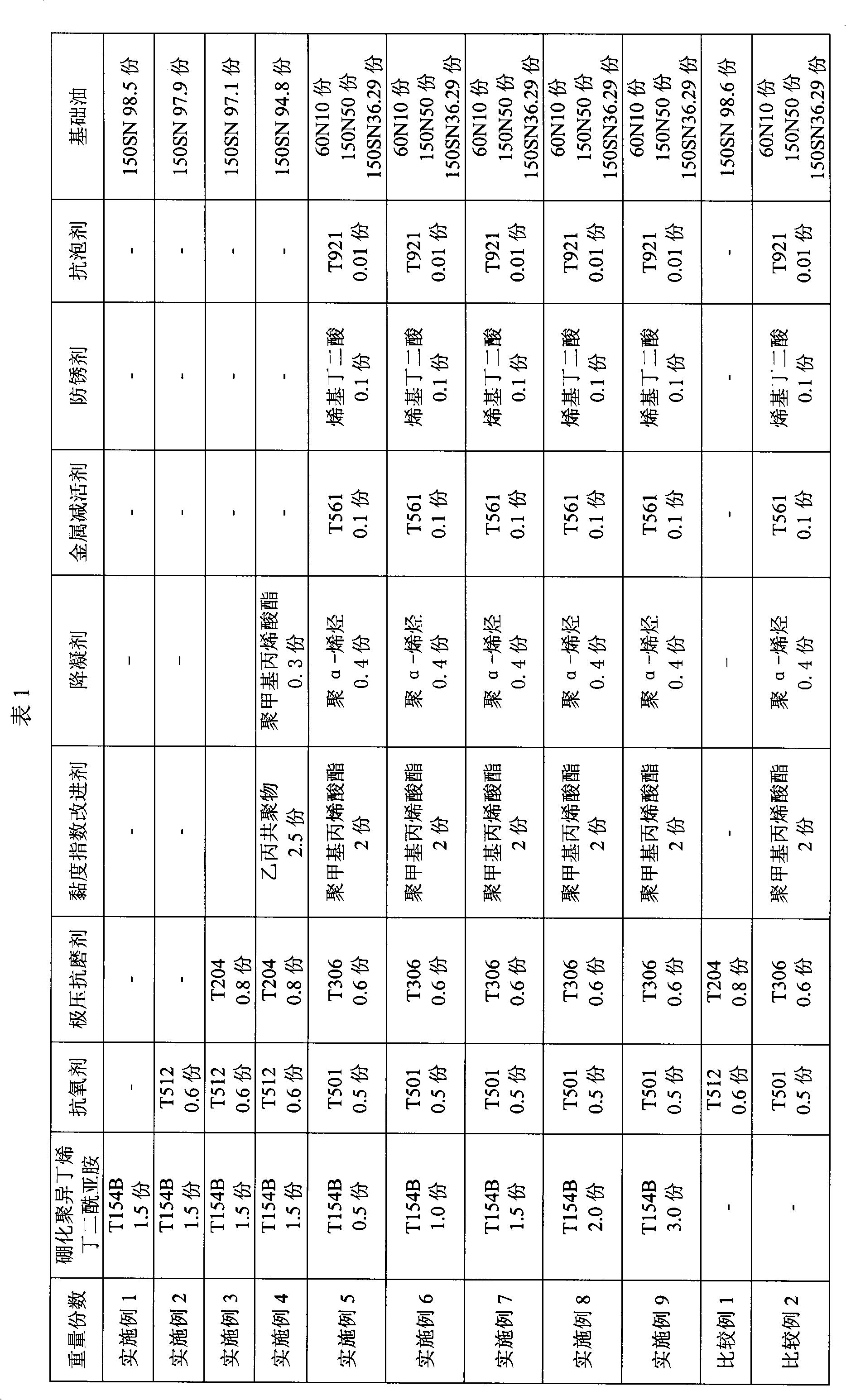
Lubricating oil composition for transmissions
ActiveUS20150376544A1Excellent characteristicsMaintain good propertiesLiquid carbonaceous fuelsLubricant compositionHeat resistancePhosphorus acid
A lubricating oil composition for transmissions having improved fuel saving properties, excellent metal fatigue prevention properties, and heat resistance containing: a lubricating base oil containing (A) a mineral base oil having a 100° C. kinematic viscosity of 1.5 mm2 / s or higher and 3.5 mm2 / s or lower, a pour point of −25° C. or lower, a viscosity index of 105 or greater, a % Cp of 85 or greater, a % CN of 2 or greater and 20 or less and a % CA of 3 or less and (B) a monoester-based base oil having a 100° C. kinematic viscosity of 2 to 10 mm2 / s; (C) a phosphorus acid ester; and (D) a boronated ashless dispersant, the ratio of the mass percent of boron to the mass percent of phosphorus in the composition (B / P) being from 0.07 to 0.42, and the composition having a 100° C. kinematic viscosity of 2.5 to 4.0 mm2 / s is described.
Owner:JX NIPPON OIL & ENERGY CORP +1
Efficient and energy-saving repairing agent
The invention relates to an efficient, energy-saving and wear-resistant repairing agent with extreme pressure resistance (strong bearing capacity) and strong wear resistance, which greatly improves the performances of lubricating oil and lubricating grease, also greatly reduces energy consumption and mechanical wear and has a function of automatically repairing slight mechanical wear. The efficient, energy-saving and wear-resistant repairing agent with extreme pressure resistance and strong wear resistance comprises a coupling agent, a wear resistant agent, a lubricating agent, a reaction accelerant, a repairing factor and a dispersant and concretely comprises 0.5-2% of aluminium compound, 0.25-2% of calcium compound, 0.25-2% of magnesium compound, 0.25-2% of molybdenum compound, 1-6% of boronized amide, 0.5-5% of the coupling agent, 0.25-2% of the reaction accelerant, 50-95% of stabilizing agent and the dispersant. 3-5% of engine oil is added in the lubricating oil of an engine or gear. Detection results are as follows: the extreme pressure resistance is up to 108kg and is remarkably increased by three grades, the friction coefficient is reduced as comparison with the friction coefficient during no addition and is up to 55.6%, and the wear resistant effect is remarkable. During practical running tests of various vehicles, the oil saving efficiency is up to 11.0-20.90%.
Owner:ANHUI FANYA ENERGY
Directional power-assisted oil composition and its application
ActiveCN102337172AMaintain viscosity temperatureImprove anti-corrosion performanceAdditivesChemical compositionBoron
The invention relates to a directional power-assisted oil composition and its application and mainly solves the problems of poor compatibility between a directional power-assisted oil and a rubber sealing member and the fault of a power steering system due to deformation or failure of the rubber sealing member with the application of a power-assisted oil composition in the power-assisted steering system in the prior art. The directional power-assisted oil composition comprises the following components of: by weight, a) 0.1-5 parts of boron polyisobutylene succinimide; b) 95-99.9 parts of base oil. By the adoption of the technical scheme, the problems are greatly solved. In addition, the technical scheme can be used in the industrial production of the directional power-assisted oil composition provided by the invention.
Owner:CHINA PETROLEUM & CHEM CORP
Nonaqueous electrolytic secondary battery and method of producing anode material thereof
InactiveUS7238449B2Final product manufactureActive material electrodesMetallic lithiumElectrical battery
A nonaqueous electrolyte secondary battery having a positive electrode made of a carbonaceous material, an electrolyte containing a lithium salt, and a negative electrode made of metallic lithium or a material capable of occluding and releasing lithium, wherein said positive electrode is formed from a boronized graphitic material containing boron or a boron compound such that the content of boron therein is 0.05-11 wt %. A method for production of the positive electrode of the nonaqueous electrolyte secondary battery.
Owner:FDK CORP
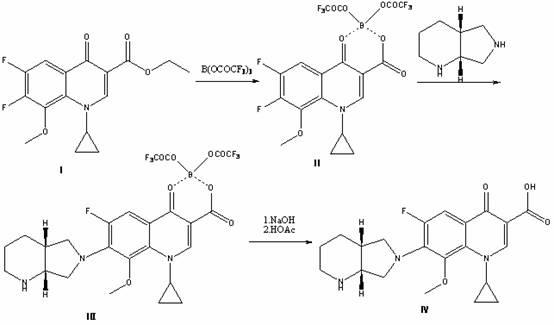
High selectivity method for synthesizing moxifloxacin
ActiveCN102351858AAvoid it happening againSimple processing methodOrganic chemistryAcetic anhydrideIce water
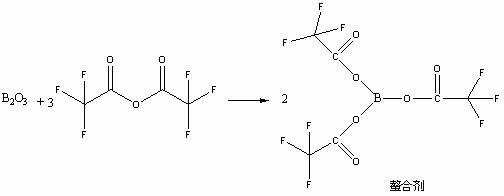
The invention discloses a high selectivity method for synthesizing moxifloxacin. The method comprises the following steps of: reacting boric anhydride with trifluoro acetic anhydride to obtain a chelant; reacting 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester with the chelant, cooling to room temperature, adding ice water, performing suction filtration, and washing a filter cake with water until neutrality to obtain a 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoroacetic anhydride boronized chelate; and reacting the 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoroacetic anhydride boronized chelate with (S,S)-2,8-diazabicyclo[4,3,0]nonane to obtain a 1-cyclopropyl-6-fluoro-7-([S,S]-2,8-diazabicyclo[4,3,0]nonane-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid ethyl ester trifluoroacetic anhydride boronized chelate, recycling a solvent under reduced pressure, adding alkali, refluxing, discoloring, filtering, freezing, performing suction filtration, and drying a filter cake. The method is simple, mild in conditions, and high in selectivity, avoids difficultly separated impurities, is high in reaction yield and product purity, and is suitable for industrial production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Method for preparing crystalline transitional metal boride - cobalt boride
InactiveCN102442706ANovel synthetic methodSimple processCobalt compoundsMetal/metal-oxides/metal-hydroxide catalystsPhysical chemistryCobalt boride
The invention relates to a new method for preparing crystalline transitional metal boride CoB (cobalt boride) with oxide or hydroxide of cobalt and borohydride of cobalt through solid phase reaction. The method provided by the invention comprises the steps as follows: uniformly mixing oxide or hydroxide of cobalt and borohydride of cobalt according to the molar ratio of 1:1 to 1:1.5, tabletting under the pressure of 15 to 20 MPa, keeping the temperature of 400 to 800 DEG C for 6 to 24 hours under the protection of argon gas, and cooling to the ambient temperature slowly; and the obtained product is washed fully with deionized water and alcohol and then vacuum dried, thus the product CoB can be obtained. The method has the characteristics that the synthetic method is novel, the adopted process is simple, and pure crystalline CoB can be obtained without high temperature, high pressure or other complicated procedures; and the used raw materials are simple and easy to obtain, and the cost is low, so that the method is applicable to mass production.
Owner:NANKAI UNIV +1
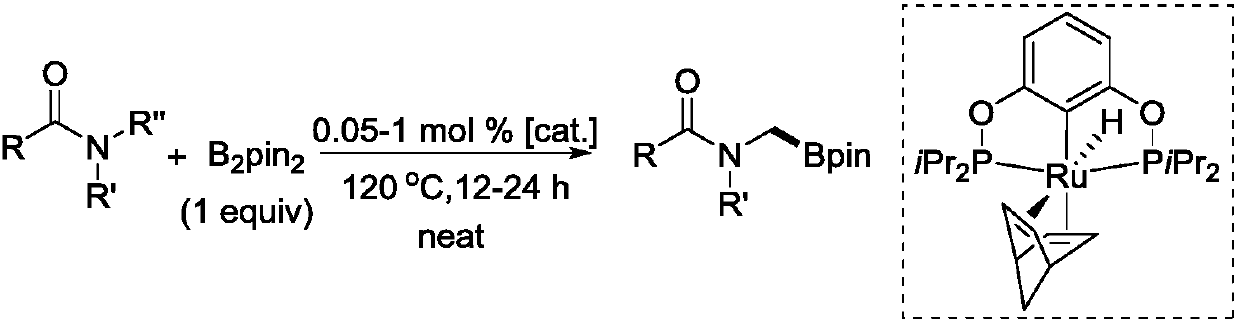
Novel method for ruthenium-catalyzed selective boronation reaction of amides
ActiveCN107892698AGroup 3/13 element organic compoundsFunctional group formation/introductionOrganic synthesisOrtho position
The present invention relates to a novel method for ruthenium-catalyzed selective boronation reaction of amides. According to the method, a hexacoordinated metal ruthenium complex containing a norbornadiene (NBD) ligand is taken as a catalyst, an N,N-disubstituted amide and bis(pinacolato)diboron are taken as reaction substrates, and in the absence of reaction solvents and under mild reaction conditions, a carbon-hydrogen bond of methylene at the ortho position of a nitrogen atom in the N,N-disubstituted amide is selectively subjected to a boronation reaction under efficient catalysis, so thata corresponding amide borate product is obtained. Compared with currently reported methods, the novel method generally has the advantages of wide substrate applicability, low catalyst usage amount, simple operation and the like. The method of the present invention realizes for the first time a selective C(sp3)-H boronation reaction of N,N-dimethyl substituted aromatic amide derivatives. In addition, the method realizes for the first time a metal-ruthenium-catalyzed selective dehydro-boronation reaction of N,N-disubstituted amides, and provides a completely new reaction strategy for organic synthetic intermediates of amide borates.
Preparation method of rutile type boron-doped titania (B-TiO2) microsphere with exposed high energy crystal face {001}
InactiveCN103657625AAvoid profileAvoid stabilityPhysical/chemical process catalystsHigh energyMicrosphere
The invention provides a preparation method of a rutile type boron-doped titania (B-TiO2) microsphere with an exposed high energy crystal face {001} and belongs to the technical field of the preparation of inorganic material. The preparation method comprises the following specific steps: adding titanium boride (TiB) into a hydrochloric acid solution hydrazine containing natrium fluoride (NaF) to obtain a mixed solution, forcefully agitating, then transferring the mixed solution to a reactor with a polytetrafluoroethene lining, keeping the mixed solution at a constant temperature of 200 DEG C for 12-24 hours, naturally cooling the mixed solution to the room temperature so as to obtain a product, filtering the product, respectively washing and settling the product with distilled water and absolute ethyl alcohol for three times, drying the product at a temperature of 60-80 DEG C for 12-24 hours, and finally preparing the B-TiO2 microsphere with the exposed high energy crystal face {001}, of which exposing rate nearly reaches 100 percent. The preparation method is simple in preparation technology and good in repeatability; the prepared B-TiO2 microsphere is controllable and uniform in size and has a diameter of 3-5 micron; the element boron is doped, and is uniform in distribution and controllable in the amount of dopping; in addition, the prepared B-TiO2 microphere has excellent visible light catalytic activity, and is likely to be widely applied in the fields of photolytic water hydrogen preparation, organic contaminant degradation and the like.
Owner:ZHANJIANG NORMAL UNIV
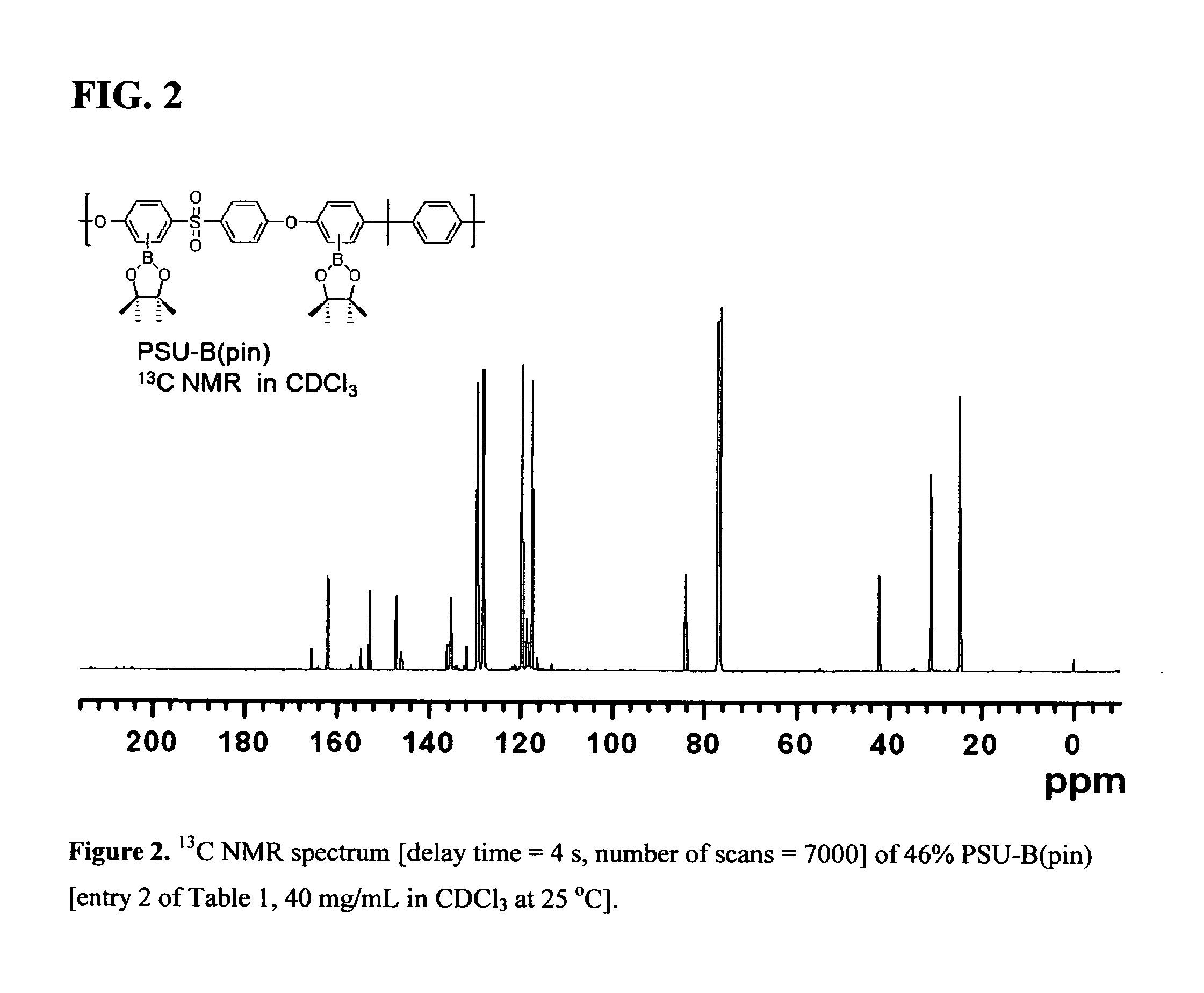
Polyaryletherketone containing boric acid ester, azo polyaryletherketone and preparation method thereof
The invention discloses polyaryletherketone containing a boric acid ester, azo polyaryletherketone and a preparation method thereof, and belongs to the technical field of preparation of a high polymer material. The method comprises the following steps: firstly, synthetizing bisphenol A polyaryletherketone, and carrying out catalytic boriding reaction under a 1,5-cyclooctadiene chloride iridium dipolymer by using the polyaryletherketone and boron pinacol ester; secondly, synthetizing a 4-iodine-4'-(N,N-dimethyl amine) azobenzene monomer; finally, reacting with the 4-iodine-4'-(N,N-dimethyl amine) azobenzene monomer under catalysis of tetrakispalladium by using a polyaryletherketone material containing the boric acid ester, so as to prepare the azo polyaryletherketone. By adopting the method, an azo monomer can be successfully led to the polyaryletherketone, but the performance of the polyaryletherketone is not affected, biphenyl structure azo polyaryletherketone with stable performance also can be generated, and the storage stability of the azo material can be improved. Therefore, the method is expected to be applied in the aspect of preparation of a novel azo polymer material.
Owner:JILIN UNIV
Ultrathin nanosheet material with coexisting crystal and amorphous interfaces and water electrolysis application of ultrathin nanosheet material
ActiveCN112439459AFully exposedFast transferMaterial nanotechnologyOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystOxygen evolution
The invention relates to an ultrathin nanosheet material with coexisting crystal and amorphous interface and application of the material. A preparation method of the material comprises the following steps: 1) pretreating foamed nickel, and taking the pretreated foamed nickel as a matrix; 2) mixing a nickel nitrate solution with a dimethylimidazole solution to obtain a mixed solution; 3) putting the matrix into the mixed solution, and carrying out hydrothermal reaction to obtain a nickel-based zeolite imidazate skeleton; and 4) washing and drying the nickel-based zeolite imidazate skeleton, putting the nickel-based zeolite imidazate skeleton into a sodium borohydride solution, and carrying out a boronation reaction to obtain the crystal and amorphous interface coexisting ultrathin nanosheetmaterial. The material is used as a catalyst for water electrolysis reaction. Compared with the prior art, the crystal and amorphous interface coexisting ultrathin nanosheet electrocatalyst materialprepared by the invention not only has excellent electrocatalytic performance and multiple active sites, but also is low in cost, can be used for carrying out stable and efficient hydrogen evolution reaction, oxygen evolution reaction and total water decomposition under different current densities, and has a wide application prospect.
Owner:FUDAN UNIV
Method for high-purity and high-yield preparation of p-chlorophenylboronic acid
InactiveCN106946915ALow costReduce manufacturing costGroup 3/13 element organic compoundsGrignard reagentChloride
The invention discloses a method for high-purity and high-yield preparation of p-chlorophenylboronic acid. The method comprises: (1) carrying out a Grignard reaction on magnesium chips and 1,4-dichlorobenzene under the action of an initiator to obtain a 4-chlorophenyl magnesium chloride Grignard reagent; and (2) carrying out a deep low temperature reaction on the 4-chlorophenyl magnesium chloride Grignard reagent obtained in the step (1) and trialkyl borate, carrying out acidolysis, and treating to obtain p-chlorophenylboronic acid. With the technical scheme of the present invention, the raw material cost and the production cost are substantially reduced, the reaction effect is good, the conversion rate during the boronization reaction is increased, the generation of the by-product impurities is reduced, the post-treatment is simple, the product purity is high, and the environmental pollution is reduced.
Owner:安徽至善新材料有限公司
Synthesis method of cycloalkene-1-boronic acid pinacol ester
InactiveCN104478918AAvoid distillationAvoid the problem of impurity generation that is difficult to control when the temperature risesGroup 3/13 element organic compoundsCycloalkeneBiochemical engineering
The invention discloses a synthesis method of cycloalkene-1-boronic acid pinacol ester. The synthesis method comprises three reaction steps of enabling cycloketone to react with phosphorus pentachloride to obtain 1-cycloolefin chloride, preparing cycloalkene-1-boronic acid through 1-cycloolefin chloride, lithium metal and a boronizing agent by the one-step method, and enabling cycloalkene-1-boronic acid to react with pinacol to obtain the final product. According to the method, the reaction is under mild conditions, 1-cycloolefin chloride can directly enter the next reaction without distilling and purifying, and intermediates and products obtained in each step do not need distilling, so that the operation is simple, and moreover, the raw materials are low in cost and easy to obtain, and as a result, mass production can be performed.
Owner:DALIAN NETCHEM CHIRAL TECH
Silicon boron polymer and preparation method therefor and application thereof
ActiveCN105175732AIncrease viscosityInhibit and solve the problem of too fast responseTwo stepMethyl group
The invention discloses a silicon boron polymer and a preparation method therefor and an application thereof. The preparation method for the silicon boron polymer comprises two steps: firstly carrying out a reaction between 30-40 parts of a boron-containing compound and 20-30 parts of polyol to prepare a polyol ester; and then carrying out a reaction between 3-20 parts of the boronized polyol ester, 1-10 parts of methylsilicone oil, 60-100 parts of hydroxyl silicone oil, 0.1-15 parts of white carbon black and 0.01-0.5 part of a plasticizer unsaturated fatty acid to obtain the silicon boron polymer. Plasticine made from the synthetic silicon boron polymer has good bounce and stretchability; the elongation at break reaches 1000-2000%; the tensile strength is just 1-2 MPa; and the T type peel strength is just 0.1-1 kN / m. The preparation method disclosed by the invention is simple in process, short in preparation period and strong in operating controllablity; the stability of product performance can be improved; and the energy consumption is reduced, so that large-scale production is further facilitated. The prepared silicon boron polymer can be widely applied to the fields of toys for children, artistic molding, industrial molding and the like.
Owner:东莞九天量子科技有限公司
Nonaqueous electrolytic secondary battery and method of producing anode material thereof
A nonaqueous electrolyte secondary battery having a positive electrode made of a carbonaceous material, an electrolyte containing a lithium salt, and a negative electrode made of metallic lithium or a material capable of occluding and releasing lithium, wherein said positive electrode is formed from a boronized graphitic material containing boron or a boron compound such that the content of boron therein is 0.05-11 wt %. A method for production of the positive electrode of the nonaqueous electrolyte secondary battery.
Owner:FDK CORP
Carbon-based ferronickel bimetallic oxygen evolution catalyst and preparation method thereof
ActiveCN112517011AImprove hydrophobicityImprove conductivityMetal/metal-oxides/metal-hydroxide catalystsPtru catalystCarbon nanotube
The invention discloses a carbon-based ferronickel bimetallic oxygen evolution catalyst and a preparation method thereof. The preparation method of the catalyst comprises the steps of: mixing carbon nanotubes and a boron-containing organic matter to obtain mixed powder, and calcining the mixed powder to obtain boronized carbon nanotubes; dispersing the boronized carbon nanotubes in water, adding anickel salt and an iron salt, performing full dipping to obtain a mixed material, conducting separation to obtain a solid substance, and performing drying to obtain precursor powder; and placing theprecursor powder in an inert atmosphere for heat treatment, and then conducting natural cooling to room temperature to obtain a target product. According to the preparation method, boron is used for modifying the surfaces of the carbon nanotubes, the reducing capacity of the surfaces of the carbon nanotubes is reduced, NiFeOOH clusters are successfully grown on the surface of the carbon nanotubes,the NiFeOOH cluster catalyst loaded by the carbon nanotubes is obtained, and the catalyst has the advantages of good conductivity, large specific surface area and the like, shows extremely high catalytic activity and has higher oxygen evolution activity. The production cost is low, and industrial application can be achieved.
Owner:UNIV OF SCI & TECH OF CHINA
Boron-induced amorphous layered double hydroxide electrocatalyst, and preparation and application thereof
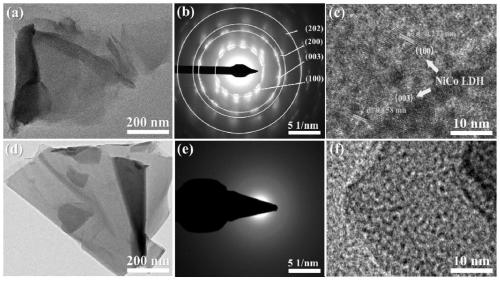
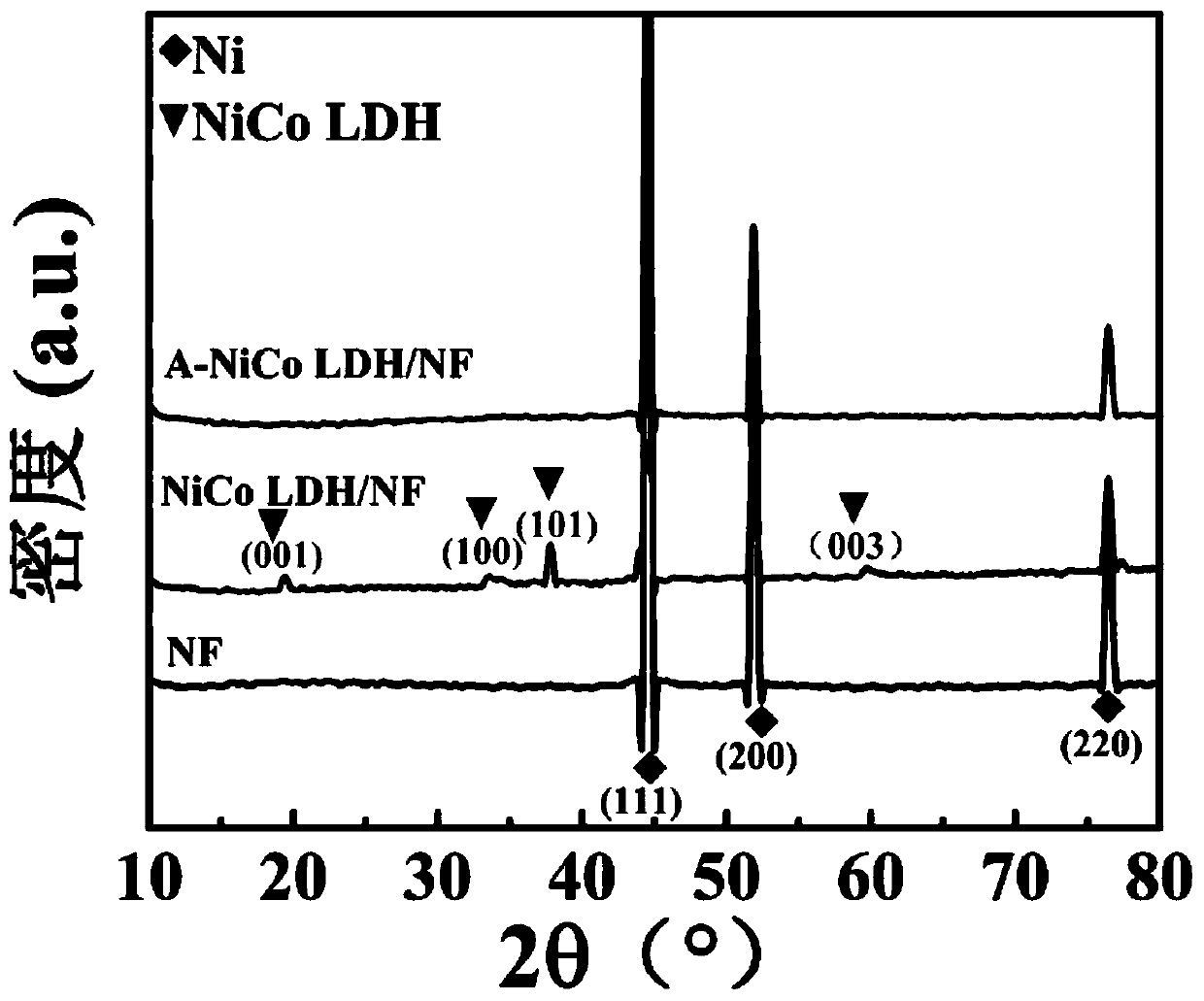
ActiveCN110124673AImprove electrocatalytic activityImprove stabilityMetal/metal-oxides/metal-hydroxide catalystsElectrodesBoron atomElectron transfer
The invention relates to a boron-induced amorphous layered double hydroxide electrocatalyst as well as preparation and application thereof. The preparation method comprises the following steps: firstly, synthesizing a transition metal layered double hydroxide nanosheet crystal precursor on a foamed nickel substrate by utilizing an in-situ growth method; and amorphizing the crystal precursor through a room-temperature boronizing method to finally prepare the self-supporting hydrogen evolution catalyst of the amorphous layered double hydroxide nanosheet. Compared with the prior art, the method for inducing the amorphization of the layered double hydroxide crystals by doping boron atoms enables the electrocatalyst to have more active sites, lower electron transfer impedance and better corrosion resistance. When the electrocatalyst is used in a hydrogen evolution reaction, the electrocatalyst can show excellent electrocatalytic activity and stability under the condition of great current density.
Owner:FUDAN UNIV
Preparation method of boriding crystalline calcium sulfonate clearing agent
InactiveCN105733745ASimple production processMeets demanding environmental assessment requirementsAdditivesSulfonateDistillation
A preparation method of a boriding crystalline calcium sulfonate clearing agent sequentially comprises the following steps of: mixing 100 parts by mass of an amorphous calcium sulfonate clearing agent and 10- 60 parts by mass of a base oil or a non-polar solvent; stirring a mixture for 5-30 minutes at the temperature between 40 DEG C and 70 DEG C; then adding 20-100 parts by mass of a polar solvent and 1-10 parts by mass of a boron reagent in order; stirring a mixture for 5-30 minutes at the temperature between 40 DEG C and 60 DEG C; then heating the mixture to the temperature between 70 DEG C and 120 DEG C and performing reaction for 0.5 to 5 hours; and continuously heating the mixture to the temperature between 120 DEG C and 200 DEG C after the reaction ends, performing distillation and removing the solvent, and obtaining the boriding crystalline calcium sulfonate clearing agent. The boriding crystalline calcium sulfonate clearing agent has a strong extreme pressure antiwear effect, can be used in the field of engine lubrication, solves the dependence of antiwear performance in oils on ZDDP, and alleviates the limitation of environmental emissions to S and P additives. The preparation method is green and environmental friendly, is high efficient and convenient, and is easy for industrialization.
Owner:PETROCHINA CO LTD
High-performance non-noble metal oxygen evolution catalyst and preparation method and application thereof
ActiveCN106423173AEasy to prepareLow costCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsBorohydrideNickel boride
The invention provides a high-performance non-noble metal oxygen evolution catalyst and a preparation method and application thereof. The high-performance non-noble metal oxygen evolution catalyst is core-shell structural nanoparticles of nickel borate-covered nickel boride, wherein a core layer is of nickel boride nanoparticles, and a shell layer is of nickel borate. A nickel salt is reduced in alkaline solution with a borohydride to obtain a precursor, and the precursor is thermally treated to obtain the non-noble metal oxygen evolution catalyst. Application of the non-noble metal oxygen evolution catalyst as anodic oxygen evolution reaction catalyst in water electrolysis equipment is also claimed in the invention. The catalyst prepared herein has excellent catalytic performance and is higher in oxygen evolution activity than other traditional non-noble metal catalysts from literature reports. The preparation method of the invention is simple, economic, convenient to perform, and easy for large-scale production, and has huge potential application value in various industrial catalyst or other fields of science.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Soluble graphene nanoribbon as well as synthetic method and application thereof
InactiveCN105502351AEasy to gatherLower bandgapMaterial nanotechnologyGraphene nanoribbonsBoronic acidDehydrogenation
The invention belongs to the technical field of functional materials and discloses a soluble graphene nanoribbon as well as a synthetic method and an application thereof. The synthetic method comprises the steps that 2,6-dibromo-4-nitroaniline and alkyl / alkoxy arylboronic acid have a Suzuki coupling reaction, 2,6-bis(4-alkyl / alkoxy benzene)-4-nitroaniline is obtained and reduced, 2,6-bis(4-alkyl / alkoxy benzene)-1,4-phenylenediamine is obtained and has halogenation, 2,6-bis(4-alkyl / alkoxy benzene)-1,4-dibromo / iodobenzene is obtained and has an Miyaura borylation reaction, and 2,6-bis(4-alkyl / alkoxy benzene)-4-boronic acid pinacol ester-1-bromo / iodobenzene is obtained; then a poly-para-phenylene derivative is generated through the Suzuki coupling reaction, and a product is obtained through oxidative dehydrogenation. The soluble graphene nanoribbon can be applied to the fields of field effect transistors, photovoltaic cells, non-linear optics and sensing.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of nano hafnium boride powder
ActiveCN113816379APlay a role in boron fixationExcellent morphologyCarbon compoundsNanotechnologyBorideAcetic acid
The invention belongs to the field of nano material preparation, and particularly relates to a novel method for preparing hafnium boride powder through a coprecipitation method. The specific process comprises the following steps: (1) dissolving HfCl4 in acetic acid to obtain a transparent solution A; dissolving boric acid and D-sorbitol in acetic acid, and stirring until the boric acid and the D-sorbitol are completely dissolved to obtain a transparent solution B; (2) after the solution B is cooled to room temperature, dropwise adding the solution A into the solution and stirring until white floccules are separated out and the solution becomes milk white; (3) drying an obtained sol; (4) fully grinding to obtain a white powdery hafnium boride precursor; and (5) calcining the hafnium boride precursor at a high temperature to obtain the nano hafnium boride powder. The preparation method is easy to operate, conditions are easy to control, and the production period is short; the prepared hafnium boride powder has nano-scale particle size and uniform distribution, and has good morphological characteristics, ultrahigh purity and high yield. And a technical basis is provided for realizing engineering and industrial preparation of high-performance, high-strength and ultrahigh-temperature ceramic materials.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Preparation method of hydroxyphenylboronic acid
ActiveCN111072698AEasy to protectEasy to realize industrial scale-upGroup 3/13 element organic compoundsBulk chemical productionGrignard reagentHydrolysis
The invention discloses a preparation method of hydroxyphenylboronic acid, which belongs to the technical field of boric acid synthesis in medical intermediates. The method comprises the following steps: starting from bromophenol, carrying out BOC, trimethylsilyl or benzyl protection, forming a Grignard reagent, reacting with borate, or carrying out one-pot reaction with borate and n-butyllithium,and hydrolyzing to obtain hydroxyphenylboronic acid. According to the invention, cheap and easily available protecting groups are adopted, so that the protecting groups are easy to remove during boronation reaction hydrolysis, industrial amplification is easy to realize, batch production is carried out on the scale of dozens of kilograms, and the process stability is good.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
Preparation method of bononized lubricating oil dispersing agent
The invention belongs to the technical field of lubricating oil additives, and particularly relates to a preparation method of a bononized lubricating oil dispersing agent. The method comprises the following steps: reacting alkenyl-terminated polyisobutyldiene and maleic anhydride to obtain polyalkylene butanedioic anhydride, heating, adding an initiator, stirring uniformly, adding methacrylate, reacting, and separating an intermediate product A; heating the intermediate product A and toluene, adding tetraethylenepentamine, reacting the intermediate product A with the tetraethylenepentamine, removing the solvent after the reaction finishes, and collecting a product B; and heating the product B, adding a bononizing agent, an accelerator and a solvent, heating to react, and recovering the solvent, thereby obtaining the bononized lubricating oil dispersing agent. The generated product has the advantages of no boric acid precipitation, high temperature resistance, favorable oxidation resistance, low micelle concentration, favorable dispersibility, favorable wear resistance under extreme pressure conditions, simple technique, no need of special equipment in the production process, and low production cost; and the solvent used in the bononizing process can be recovered and recycled.
Owner:临沂星火知识产权服务有限公司
Marine diesel engine oil composition and application thereof
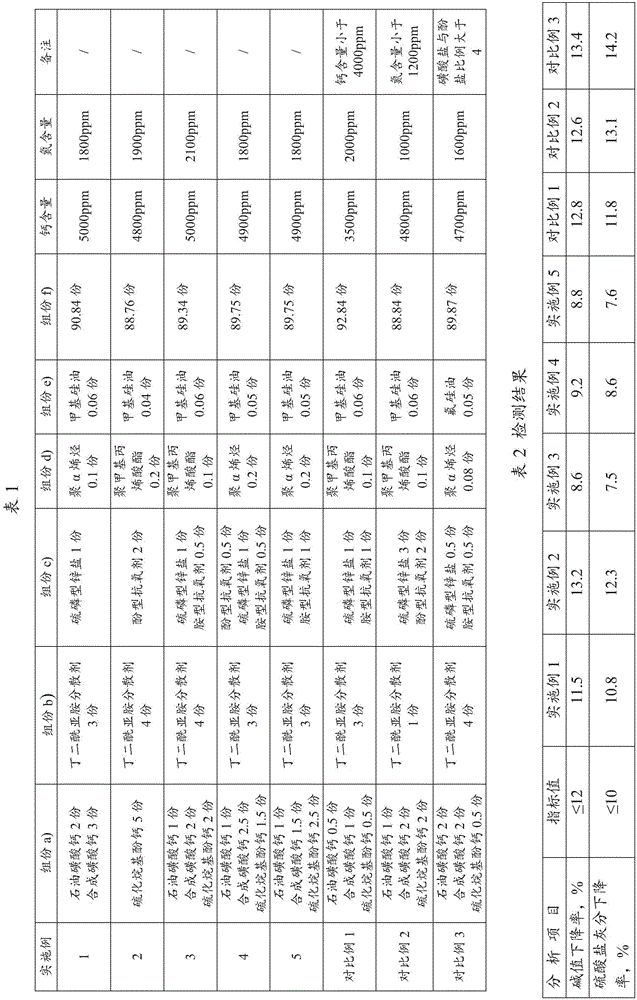
The invention relates to a marine diesel engine oil composition and application thereof; the composition is prepared from, by weight, 1-15 parts of at least one of sulfonate and sulfurized alkyphenate a); 1-10 parts of at least one of polymer ashless dispersant, succinimide dispersant or boronized ashless dispersant b); 1-10 parts of at least one of amine antioxidant, phenol antioxidant, thiophene antioxidant or carbamate antioxidant c); 0.05-1 part of at least one of poly-alpha-olefin or polymethacrylate d); 0.01-1 part of at least one of methyl silicone oil or fluorosilicone oil anti-foaming agents e); 50-95 parts of at least one of HVI type I basic oil or HVI type II basic oil; the composition is not less than 4000 ppm in calcium content and not less than 1000 ppm in nitrogen content. The composition is applicable to the lubrication of marine engines.
Owner:CHINA PETROLEUM & CHEM CORP
Method for preparing cyclopropyl boronic acid
ActiveCN105001249AReduce usageEasy to operateGroup 3/13 element organic compoundsOrganic baseBoronic acid
The invention discloses a method for preparing cyclopropyl boronic acid. Cyclopropyl methanoic acid is adopted as a raw material and added into a solution obtained after n-butyllithium reacts with organic alkali at the low temperature, and then a boronizing reagent is added into the mixture; after boronation is finished, acid is added for quenching to obtain 1-carboxyl cyclopropyl boronic acid; the intermediate is added into high-boiling-point solvent and heated until the temperature is 80 DEG C-150 DEG C, after reaction deacidification, methylbenzene is added into the mixed solution for dehydration to form cyclopropyl boronic acid trimer, and the cyclopropyl boronic acid trimer is hydrolyzed to obtain the cyclopropyl boronic acid; the melting point of the obtained cyclopropyl boronic acid is 90 DEG C-95 DEG C, and the HNMR purity of the obtained cyclopropyl boronic acid is over 98%. The method is easy to operate and suitable for industrial scale-up production, and no highly toxic chemical is used in the whole process.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
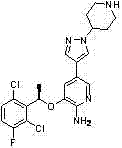
Method for synthesizing Crizotinib intermediate
InactiveCN102898449AEasy to synthesizeReduce manufacturing costGroup 3/13 element organic compoundsPtru catalystBenzoyl peroxide
The invention belongs to the technical field of medicine synthesis, and in particular relates to a method for synthesizing a Crizotinib intermediate. The method comprises steps of: a) reacting a raw material 4-mesylate piperidine-1-formic acid tert-butyl ester (2) with 4-nitro pyrazole to prepare a compound 3; b) reducing nitro by using hydrazine hydrate to obtain an amino compound 4; and c) diazotizing the compound 4 by using tert-butyl nitrite, and reacting the compound 4 with a boric acid ester compound 5 in the presence of a free radical initiator benzoyl peroxide, so as to prepare the Crizotinib intermediate (1). Compared with an existing synthesis method, the method provided by the invention has the following advantages: a diazotization method is used to synthesize the boric acid ester product; and compared with an existing Miyaura boronation method catalyzed by Pd, the method avoids the usage of expensive palladium catalyst and ligand, and has the merits of mild reaction condition, high yield, simple operation, cheap and easily available raw materials and short reaction period, and is quite easy for industrialized mass production.
Owner:TONGJI UNIV
Carbon material and method for manufacturing same
ActiveCN109311674ADoes not hinder vertical alignment propertiesImprove featuresFullerenesGraphiteCarbon nanotubePhysical chemistry
Provided are: a carbon material which has boron atoms and / or phosphorus atoms with their characteristic structures and functions intact introduced into a carbon material such as carbon nanotubes; anda method for manufacturing the carbon material. This carbon material has boron atoms and / or phosphorus atoms introduced to a proportion of the carbon atoms that constitute the carbon material, and ismanufactured by the method for manufacturing the carbon material, the method including: a step of bringing into contact a fluorination processing gas that includes a fluorine-containing gas with the carbon material to perform fluorination processing on the surface of the carbon material; and a step of bringing into contact a boronizing gas that includes a boron-containing gas with the carbon material after the fluorination processing to boronize the carbon material and / or bringing into contact a phosphorizing gas that includes a phosphorus-containing gas with the carbon material after the fluorination processing to phosphorize the carbon material.
Owner:TOHOKU UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com