Polyaryletherketone containing boric acid ester, azo polyaryletherketone and preparation method thereof
A technology of polyaryletherketone and boric acid ester, which is applied in the field of polymer material preparation, can solve the problems of polymer glass transition temperature drop, and achieve the effect of improving storage stability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
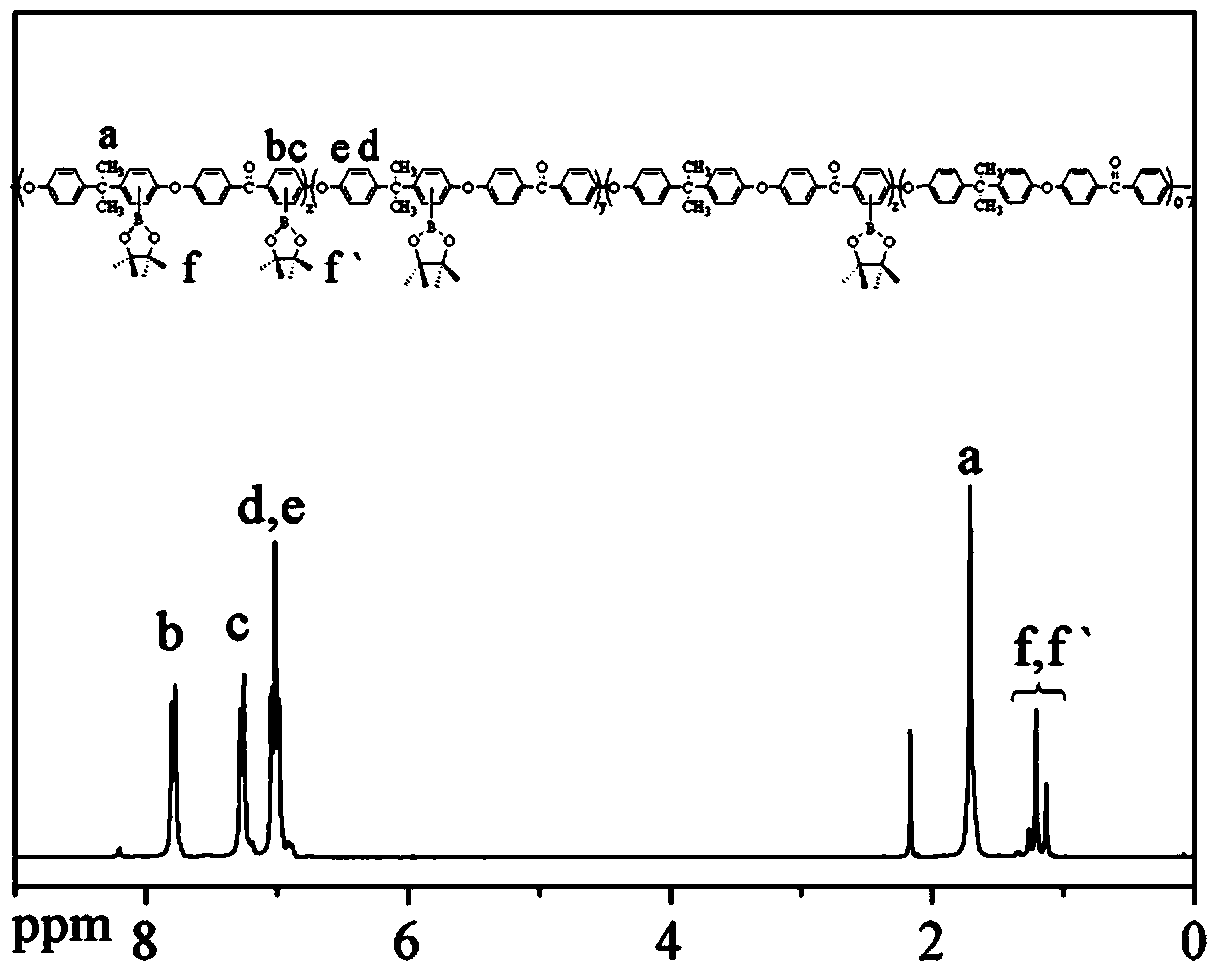
[0051] Put 13.6974g of bisphenol A monomer, 13.0920g of 4,4'-difluorobenzophenone monomer and 9.1205g of anhydrous potassium carbonate, 107mL of sulfolane, and 36mL of toluene into a In the three-necked flask of the device, pass nitrogen, start stirring, heat up to 130°C until the azeotropic dehydrating agent refluxes, react for 2 hours, remove the azeotropic dehydrating agent, heat up to 175°C and continue to react for 8 hours; Precipitation in deionized water, crushing, washing and drying to obtain bisphenol A polyaryletherketone polymer.
[0052] The molecular formula is as follows:
[0053]
[0054] Among them, m=15~25.
Embodiment 2
[0056] Firstly, the three-neck flask was baked with a gas lamp under vacuum, then flushed with nitrogen gas after cooling, put in a magnetic stirrer, and added 4.0748g (10mmol) of bisphenol A polyaryletherketone and 2.0316g (8mmol) of bisboronic acid Butyl alcohol ester, then add 0.0806g [IrCl (COD)] 2 and 0.0644g 4,4'-di-tert-butyl-2,2'-bipyridine, vacuumize and fill with high-purity nitrogen, repeat 3 times, inject 50mL tetrahydrofuran to dissolve, seal, react in an oil bath at 80°C for 12 hours; cool After dissolving in chloroform, using chloroform as solvent, filter with thin-layer chromatography silica gel (200-400 mesh), remove tetrahydrofuran by rotary evaporation, wash 5 times with acetone, and dry to obtain light yellow blocky borate-containing bisphenol A type polymer Aryl ether ketone polymer.
[0057] The molecular formula is as follows:
[0058]
[0059] Among them, n=15~25.
Embodiment 3
[0061] Weigh 21.9g (0.1mol) of 4-iodoaniline into a 250ml beaker, add 50mL of water, stir mechanically to form a paste, and add 33.6mL of concentrated hydrochloric acid (12mol / L) drop by drop. After dropping, continue to react for 1.5h and cool to -5°C. Dissolve 6.9 g of sodium nitrite in 50 mL of water, and drop this solution into the above aqueous solution of 4-iodoaniline hydrochloride at -5°C. After filtration, it was added dropwise to 200 mL of aqueous solution containing 16.4 g of sodium acetate and 12.1 g of N,N-dimethylaniline, and the temperature of the solution was controlled at -5°C. After dropping, react at room temperature for 3 to 5 hours. 24 g of aqueous sodium hydroxide solution was added dropwise to the resulting solution for neutralization. After washing with water until neutral, vacuum dry at 60°C in a vacuum oven. Recrystallize with ethanol to obtain 4-iodo-4'-(N,N-dimethylamine)azobenzene monomer in the form of orange powder.
[0062] The molecular for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com