High-performance non-noble metal oxygen evolution catalyst and preparation method and application thereof
A non-precious metal and catalyst technology, applied in the field of high-performance non-precious metal oxygen evolution catalyst and its preparation, can solve the problem that the catalyst activity needs to be improved, and achieve the effects of easy large-scale production, excellent catalytic performance and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve 5 mmoles of nickel acetate tetrahydrate in 50 ml of deionized water, add 10 ml of a mixed solution of sodium borohydride with a concentration of 1 mole per liter and sodium hydroxide with a concentration of 0.1 mole per liter, wait for about ten minutes, and the bubbles stop. Wash and centrifuge several times with deoxygenated deionized water until the pH value of the washing liquid is 7 to obtain a black powder, which is dried overnight at 60°C in vacuum to obtain a precursor; put the precursor into a porcelain boat and put it into a tube In the quartz tube of the type furnace, the air was removed with argon for half an hour, and then the temperature was raised to 350 ° C, and after heat treatment for two hours under the protection of argon, the core-shell structure nanoparticle catalyst of nickel borate-coated nickel boride was obtained.
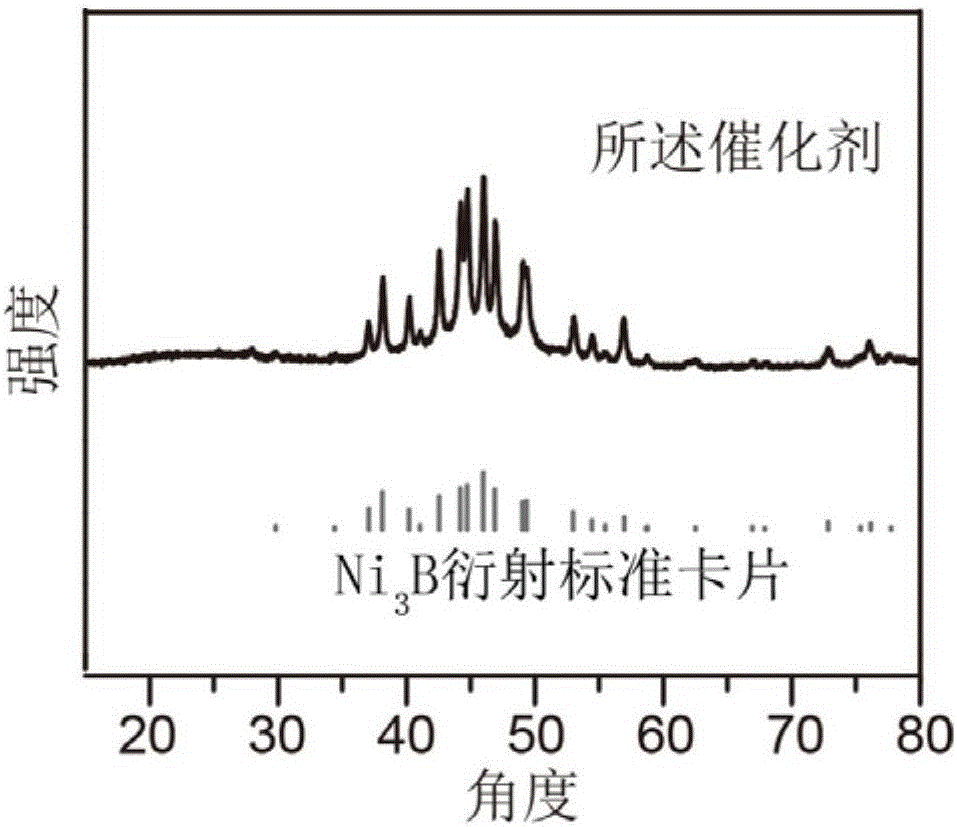
[0039] The X-ray powder diffraction curve of gained catalyst is as figure 1 shown.
[0040] It can be seen from the figu...
Embodiment 2
[0051] Prepare the non-precious metal oxygen evolution catalyst basically according to the same method as Example 1, the difference is that nickel chloride is used to replace nickel acetate as nickel salt, and the catalyst obtained is composed the same as the catalyst obtained in Example 1; The overpotential corresponding to 10 milliamperes per square centimeter obtained by testing the oxygen evolution curve in 1 liter of potassium hydroxide solution is equivalent to the overpotential obtained by the catalyst obtained in Example 1.
Embodiment 3
[0053] Prepare the non-noble metal oxygen evolution catalyst basically according to the same method as Example 1, the difference is that potassium borohydride is used instead of sodium borohydride as boron source and reducing agent, and the obtained catalyst has the same composition as the catalyst obtained in Example 1; The overpotential corresponding to 10 milliamperes per square centimeter obtained by testing the oxygen evolution curve in 1 mole per liter of potassium hydroxide solution is equivalent to the overpotential obtained by the catalyst obtained in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Overpotential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com