Furanocoumarin-Tr*ger's Base derivative as well as synthesis method and application thereof
A synthetic method and -base technology, which is applied in the field of furanocoumarin Base derivatives and their synthesis, can solve the problem of not being able to detect two metabolites at the same time, achieve good scale application prospects, high yield, and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Furocoumarins of the present invention The synthetic route of Base derivative is as follows:
[0056]
[0057] Specifically include:
[0058] (1) Preparation of Intermediate 1
[0059]Add p-bromoaniline (60mmol) and paraformaldehyde (n=3) (150mmol) into a 250mL dry round bottom flask, cool to minus 15°C, slowly add 120mL trifluoroacetic acid dropwise through a constant pressure dropping funnel, after the drop The reaction system was moved to room temperature and reacted for 7 days, quenched with ice water, adjusted the pH to neutral with ammonia water, extracted with dichloromethane (50mL×3), and the organic phase was extracted with saturated brine, dried over anhydrous sodium sulfate, and Chromatographic separation and purification (petroleum ether: ethyl acetate = 5:1), and finally recrystallized with acetone, dried to obtain intermediate 1.
[0060] (2) Preparation of Intermediate 2
[0061] Add intermediate 1 (5mmol) to a 100mL dry round bottom flask, then a...
Embodiment 2
[0366] Embodiment 2 antitumor activity:
[0367] Compounds 6 and 7 were preliminarily tested on human triple-negative breast cancer cells (MDA-MB-231), human liver cancer cells (HepG-2), human lung cancer cells (A549) and human liver immortalized cells (THLE) by MTT method. , Cytotoxicity of human bronchial epithelial cells (HBE). The results are shown in Table 2. There are three compounds (7h, 7i, 7l) at different concentrations (μg / mL), showing different degrees of inhibition and no inhibition on human bronchial epithelial cells (HBE).
[0368] Table 2. The inhibitory rate of compound 7 on cancer cells and normal cells a (IC 50 ,μg / mL)
[0369]
[0370] a IC 50 Values above 50 are marked as "-"
Embodiment 3
[0371] Example 3 Compound recognition of neuroblastoma markers homovanillic acid (HVA) and mandelic vanillic acid (VMA)
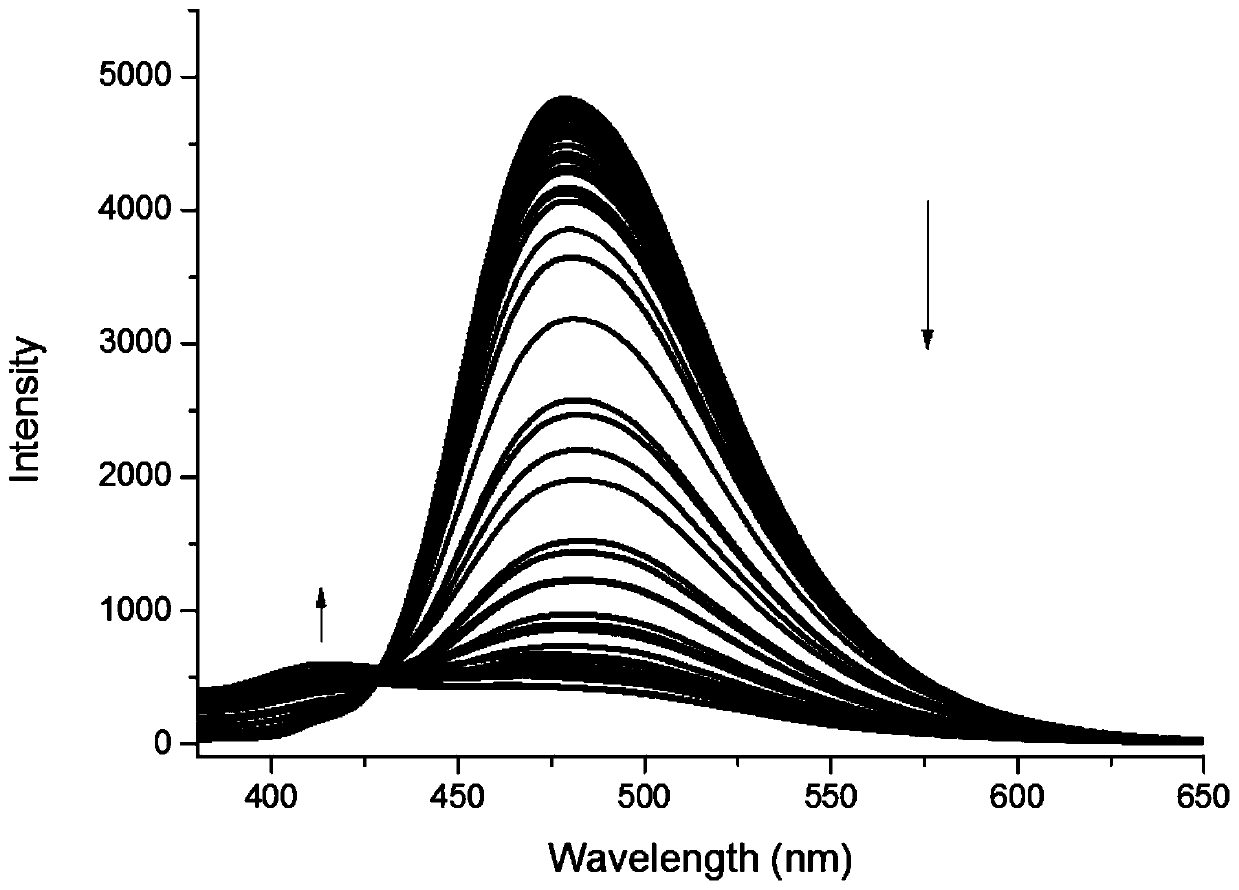
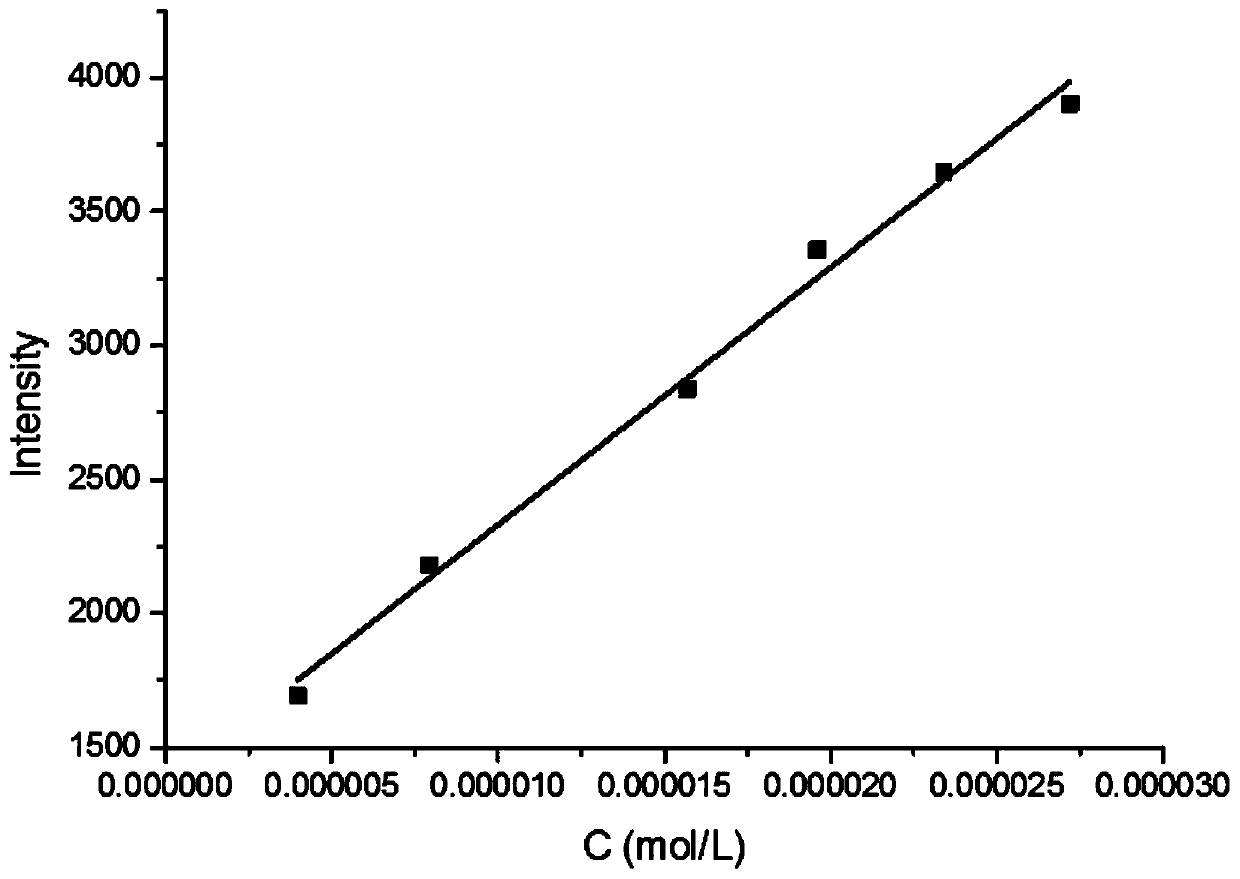
[0372] This embodiment is to test the fluorescent properties of compounds 6 and 7 to detect their recognition of neuroblastoma markers homovanillic acid (HVA) and mandelic vanillic acid (VMA). The test results are as follows: Figure 1-5 and shown in Table 3 below. Wherein, table 3 is the fluorescent property of compound 6h to HVA and VMA, figure 1 Fluorescence emission spectrum for 6h interacting with HVA and MVA, figure 2 Fluorescence emission spectra for 6h in the presence of different concentrations of HVA, image 3 is the standard curve of compound 6h to HVA, Figure 4 Fluorescence emission spectra for 6h in the presence of different concentrations of VMA, Figure 5 It is the standard curve of compound 6h to VMA. After testing, it was found that 6h has a fluorescent recognition effect on both HVA and VMA, and can be used for simultaneous qualitat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com