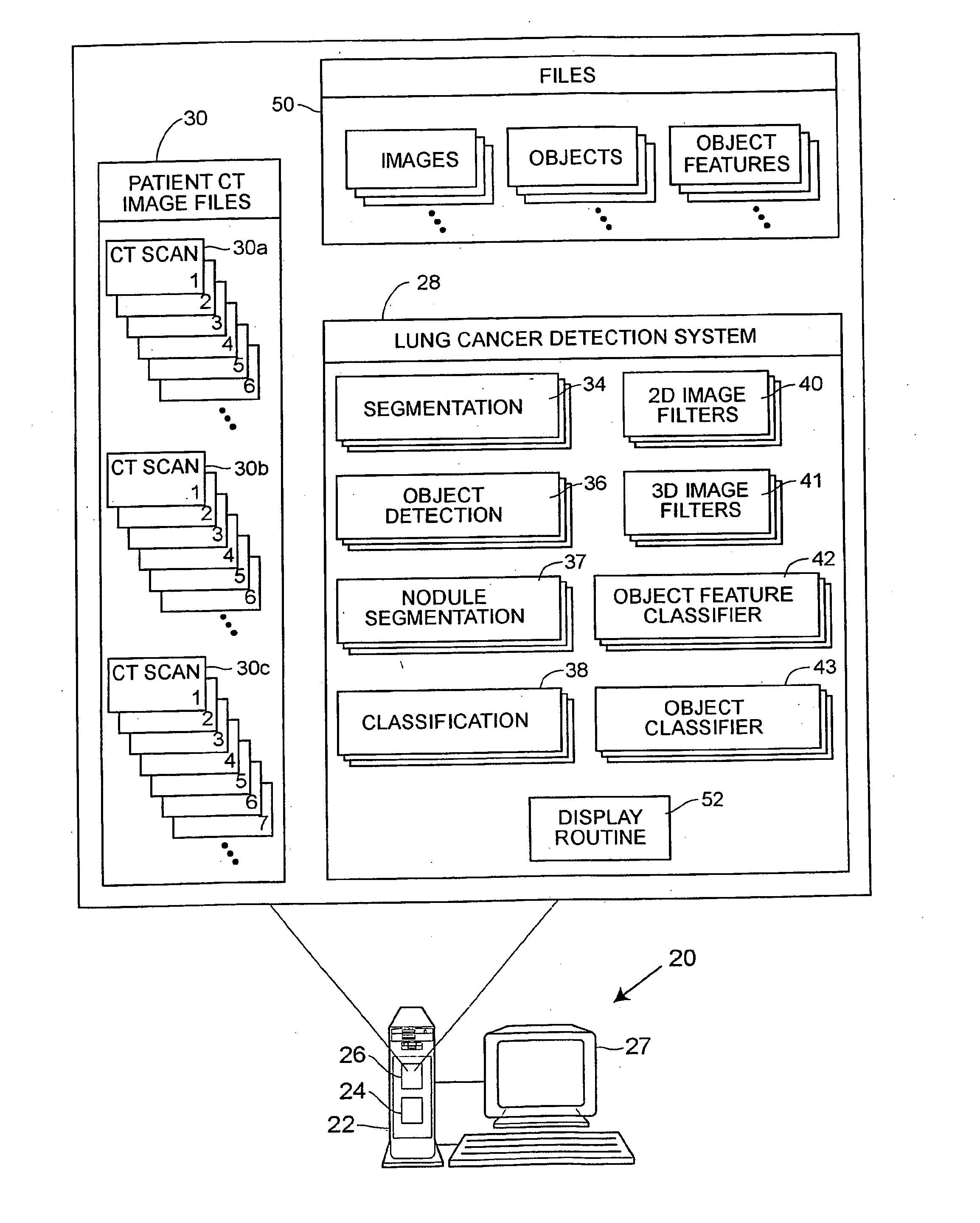
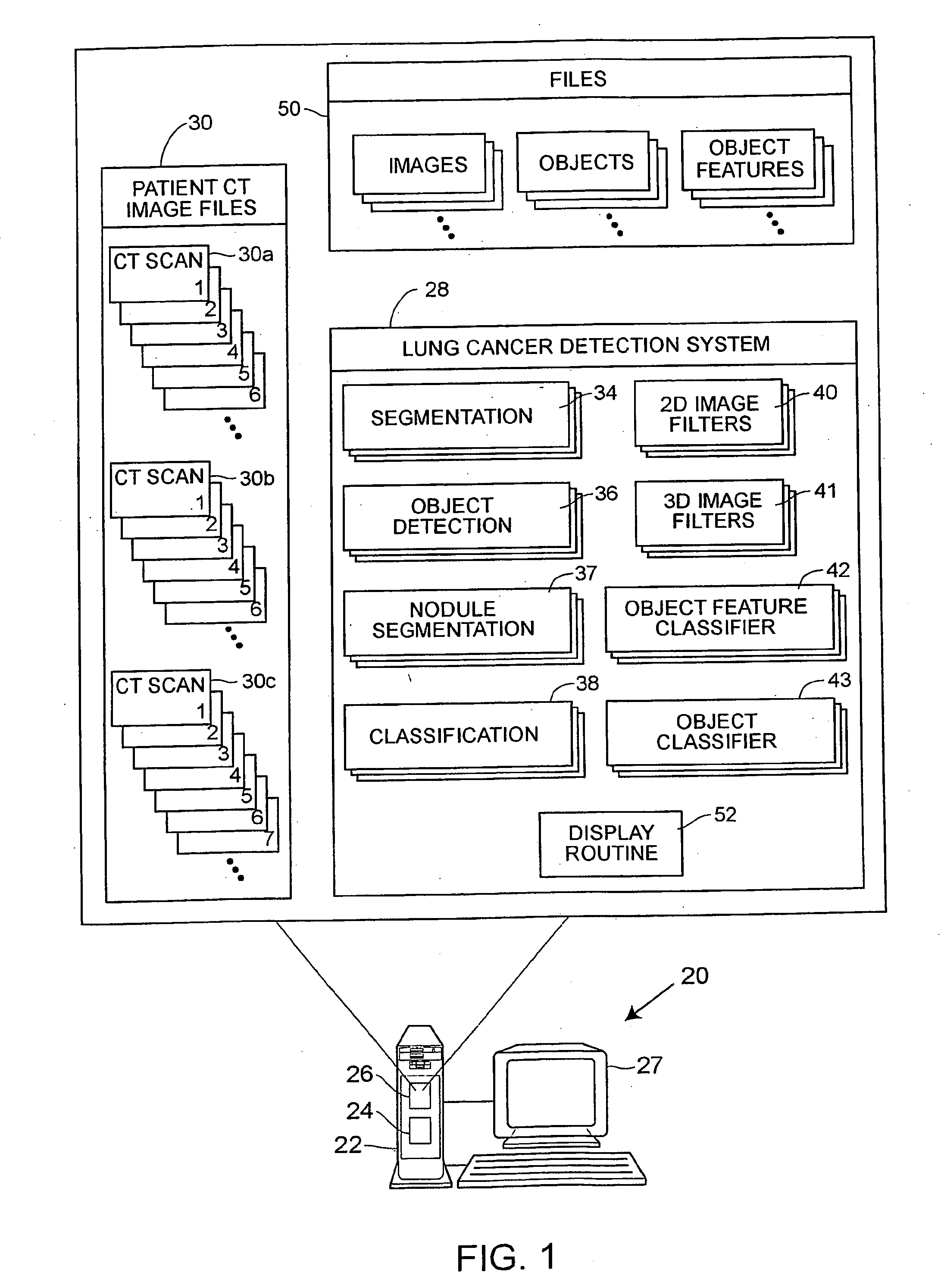
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Mediastinum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The mediastinum (from Medieval Latin mediastinus, "midway") is the central compartment of the thoracic cavity surrounded by loose connective tissue, as an undelineated region that contains a group of structures within the thorax. The mediastinum contains the heart and its vessels, the esophagus, the trachea, the phrenic and cardiac nerves, the thoracic duct, the thymus and the lymph nodes of the central chest.
Lung nodule detection and classification
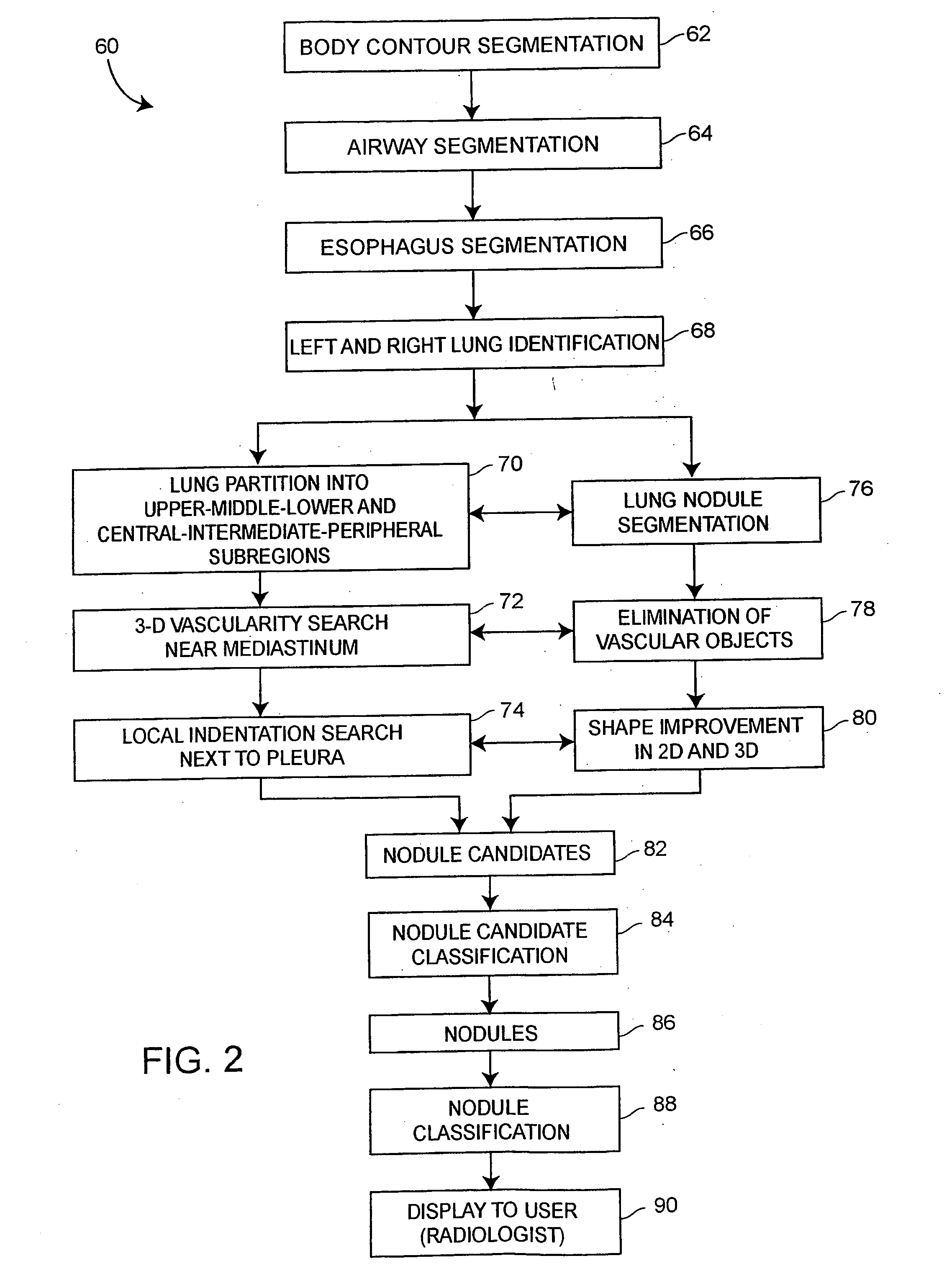
A computer assisted method of detecting and classifying lung nodules within a set of CT images includes performing body contour, airway, lung and esophagus segmentation to identify the regions of the CT images in which to search for potential lung nodules. The lungs are processed to identify the left and right sides of the lungs and each side of the lung is divided into subregions including upper, middle and lower subregions and central, intermediate and peripheral subregions. The computer analyzes each of the lung regions to detect and identify a three-dimensional vessel tree representing the blood vessels at or near the mediastinum. The computer then detects objects that are attached to the lung wall or to the vessel tree to assure that these objects are not eliminated from consideration as potential nodules. Thereafter, the computer performs a pixel similarity analysis on the appropriate regions within the CT images to detect potential nodules and performs one or more expert analysis techniques using the features of the potential nodules to determine whether each of the potential nodules is or is not a lung nodule. Thereafter, the computer uses further features, such as speculation features, growth features, etc. in one or more expert analysis techniques to classify each detected nodule as being either benign or malignant. The computer then displays the detection and classification results to the radiologist to assist the radiologist in interpreting the CT exam for the patient.
Owner:RGT UNIV OF MICHIGAN
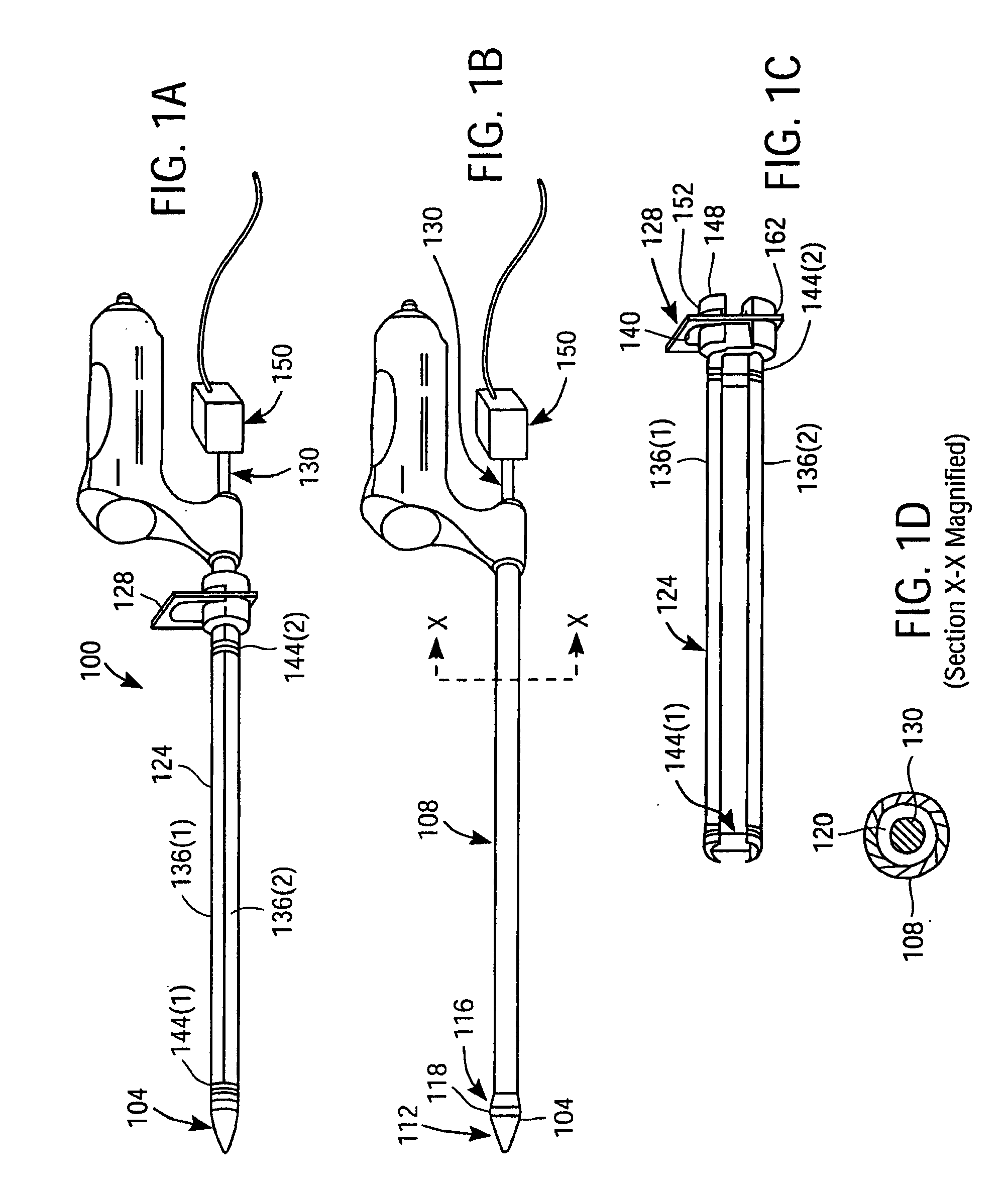
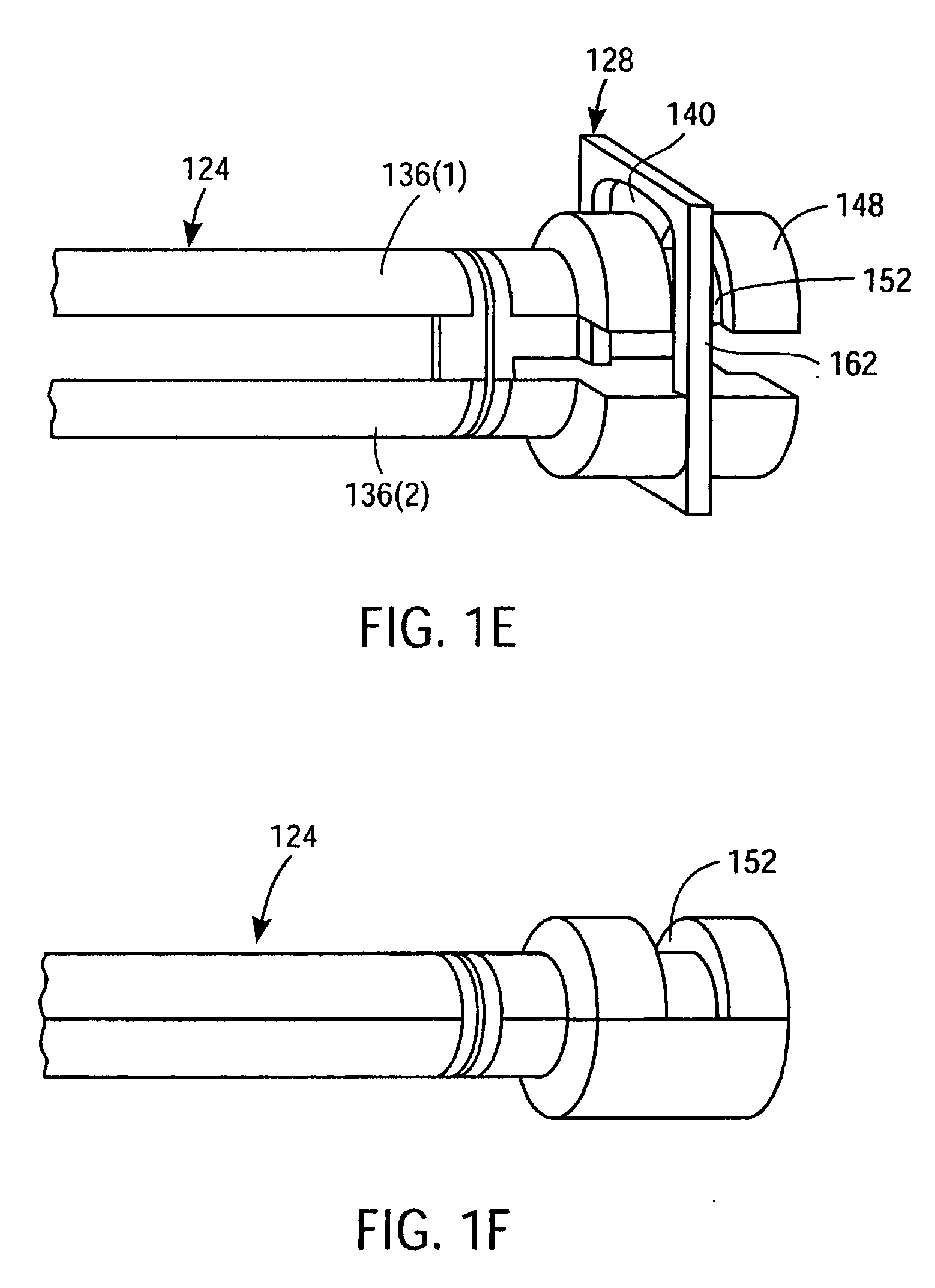
Apparatus and Method for Endoscopic Surgical Procedures
InactiveUS20080306333A1Easy to useSafe and minimally invasive accessSuture equipmentsCannulasPericardiumSurgical department
Apparatus and method for performing surgical procedures within the mediastinum and within the pericardium include an endoscopic cannula having a transparent tip, and an endoscope for introduction into the mediastinum and optionally into the pericardium via a single subxiphoid incision. A cavity may be initially dilated for advancing the endoscopic cannula using a dilating tool that exerts a lateral-expansive force against surrounding tissue for evaluating the endoscopic cannula to be introduced into the mediastinum. Other surgical instruments are positioned through the endoscopic cannula to cut a flap of the pericardium as an opening through which other surgical apparatus may be introduced. The endoscopic cannula may be swept around selected regions of the heart through an aperture near the apex of the heart to facilitate placement of epicardial tacks about regions of the heart.
Owner:MAQUET CARDIOVASCULAR LLC
Device for the extravascular recirculation of liquid in body cavities
InactiveUS7842002B2Improves liquid distributionOptimize allocationMulti-lumen catheterOther blood circulation devicesSubarachnoid spaceDouble tube
Owner:THERANOVA LLC
Device for the extravascular recirculation of liquid in body cavities
InactiveUS20060161107A1Improves liquid distributionOptimize allocationMulti-lumen catheterOther blood circulation devicesSubarachnoid spaceDouble tube
Biocompatible liquid is pumped into a body cavity (eg. subarachnoid space, peritoneum, mediastinum, pleural space) by means of one pump and removed from it by means a second pump, preferably via a double-barreled catheter is used for insertion and removal of the liquid. An optional secondary catheter removes liquid from a distal area of the body cavity. Temperature and pressure are sensed within the cavity and can be controlled by adjusting liquid flow rates. While outside the body, the liquid can be ultraviolet-sterilized, foam fractionated to remove contaminants, oxygenated and pH balanced, cooled or warmed, and augmented with exogenous liquid that may contain drugs.
Owner:THERANOVA LLC
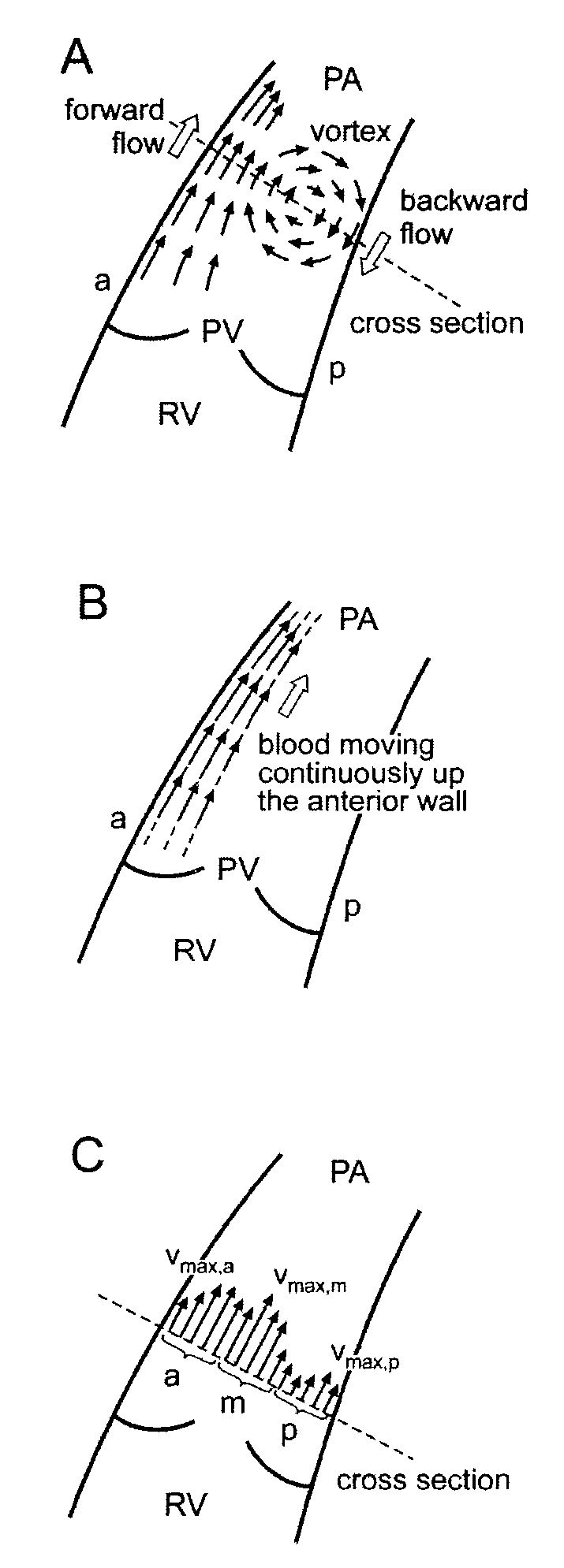
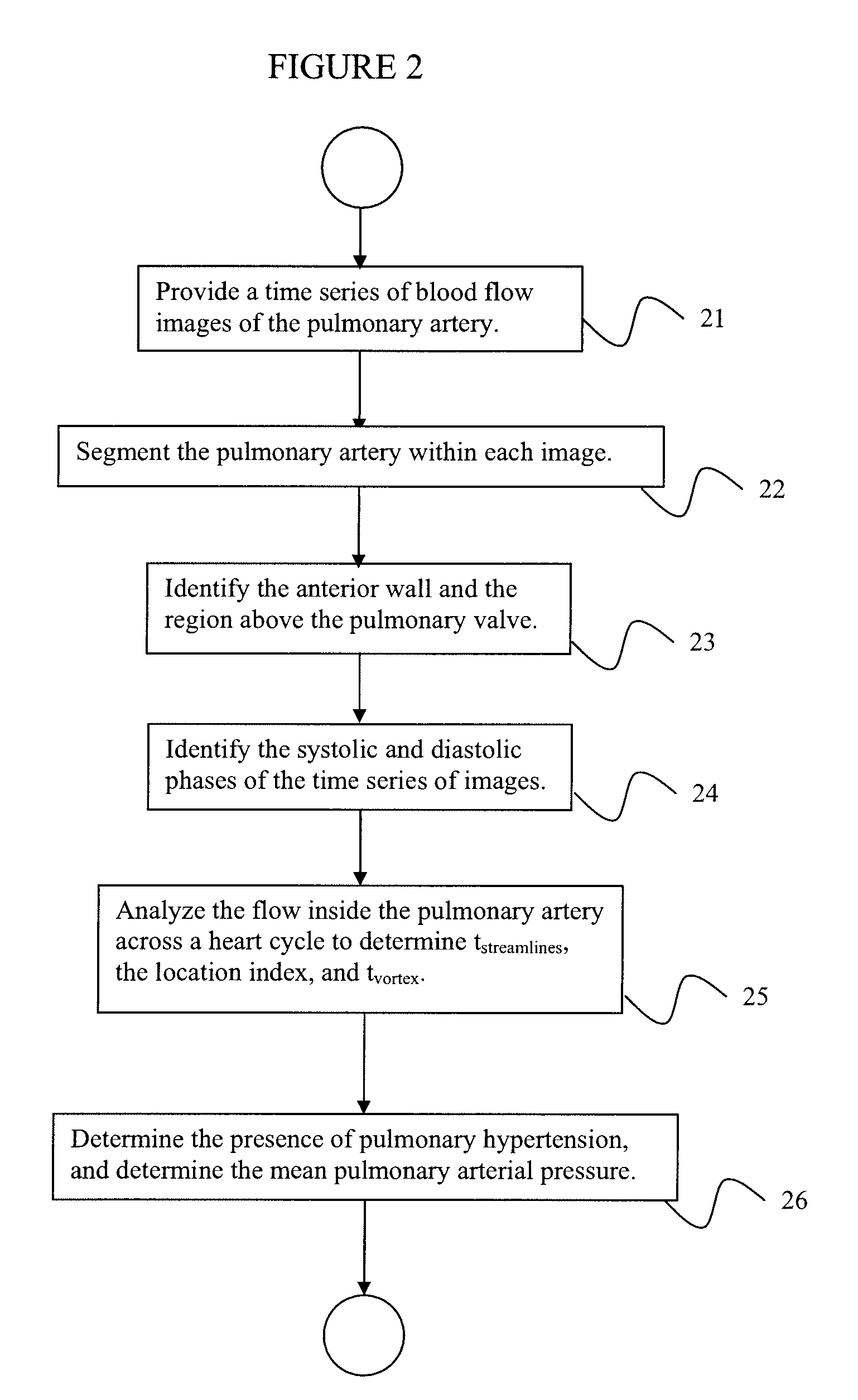
System and method for automatic, non-invasive diagnosis of pulmonary hypertension and measurement of mean pulmonary arterial pressure
InactiveUS20100094122A1Optimize workflowAnalyze moreMagnetic measurementsEvaluation of blood vesselsSystoleCardiac cycle
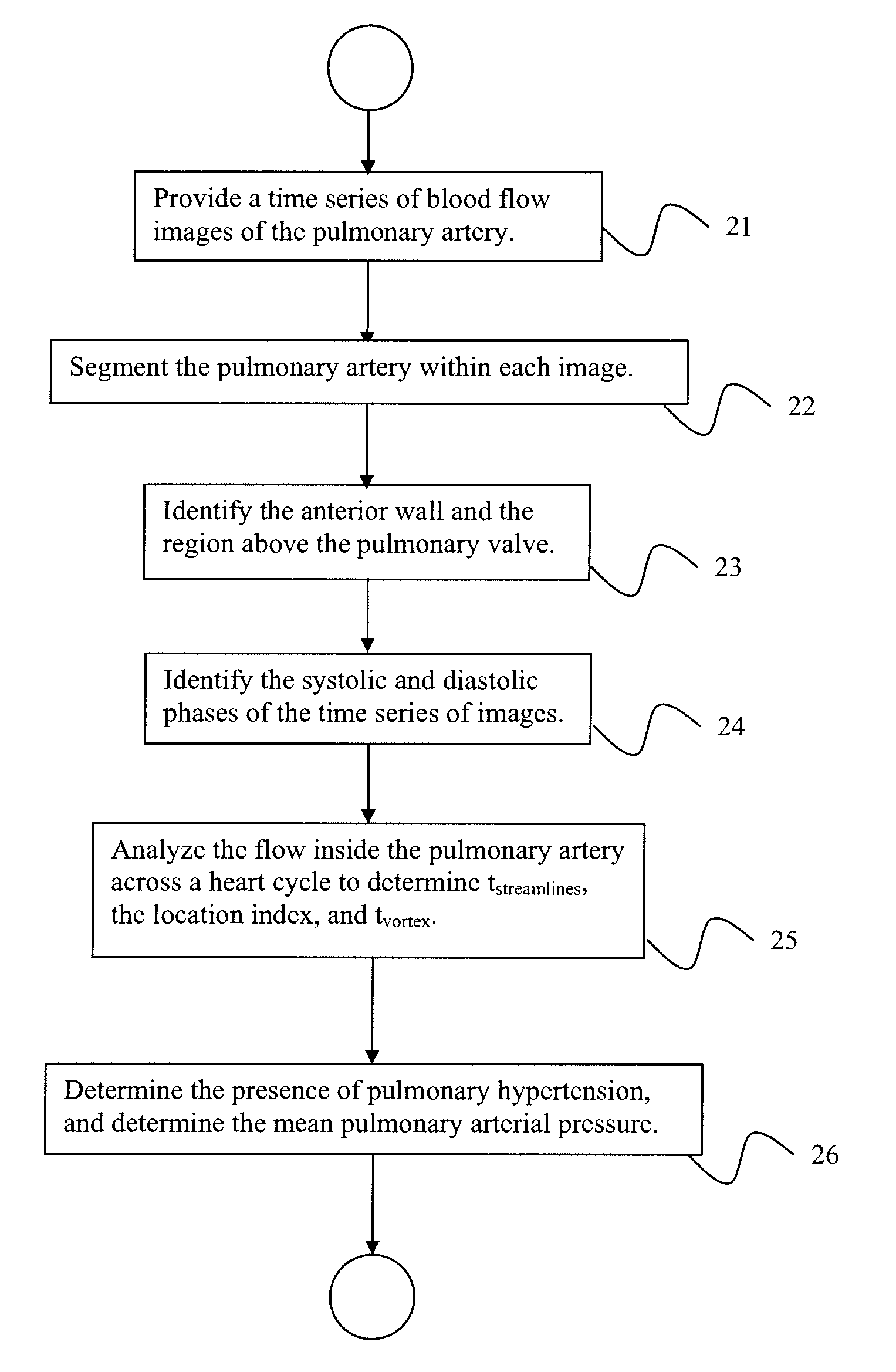
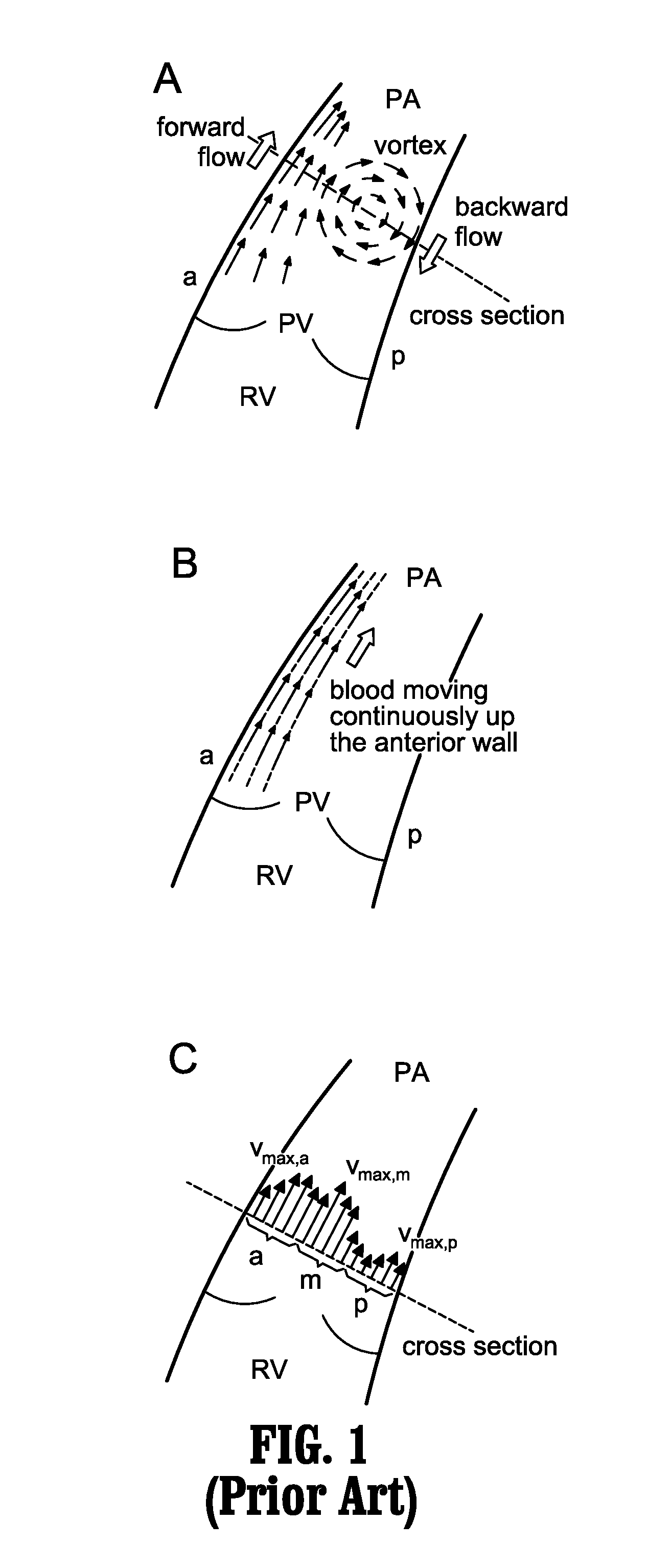
A method for diagnosing pulmonary hypertension from phase-contrast magnetic resonance (MR) images includes providing a time series of one or more magnetic resonance (MR) flow images of a patient's mediastinum during one or more cardiac cycles, segmenting the pulmonary artery within each image of the times series of images, identifying the anterior wall and pulmonary valve within the segmented pulmonary artery, analyzing blood flow during a diastolic phase of the one or more cardiac cycles to determine a relative duration of blood flow, tstreamlines, during the diastolic phase, analyzing blood flow during a latter portion of a systolic phase and a subsequent diastolic phase of the one or more cardiac cycles to detect the presence and duration tvortex of a vortex, and diagnosing the presence of pulmonary hypertension from tstreamlines and tvortex.
Owner:SIEMENS HEALTHCARE GMBH
Image segmentation, model training method and device, electronic equipment and storage medium
ActiveCN111899245BSegmentation impactImprove accuracyImage enhancementImage analysisVeinImage segmentation
The present application discloses an image segmentation and model training method and device, an electronic device and a storage medium. The image segmentation method includes: according to the to-be-segmented image including the background, the mediastinum, the arteries and the veins, obtaining the first part of the mediastinum, the arteries, the veins and the background in the mediastinal region of the to-be-segmented image. a segmentation result; according to the image to be segmented, obtain a second segmentation result of the blood vessels in the extension area of the image to be segmented and the background; according to the first segmentation result and the second segmentation result, obtain the The segmentation result of the mediastinum, the artery, the vein and the background of the image to be segmented can improve the accuracy and efficiency of segmentation between the artery and the vein.
Owner:INFERVISION MEDICAL TECH CO LTD
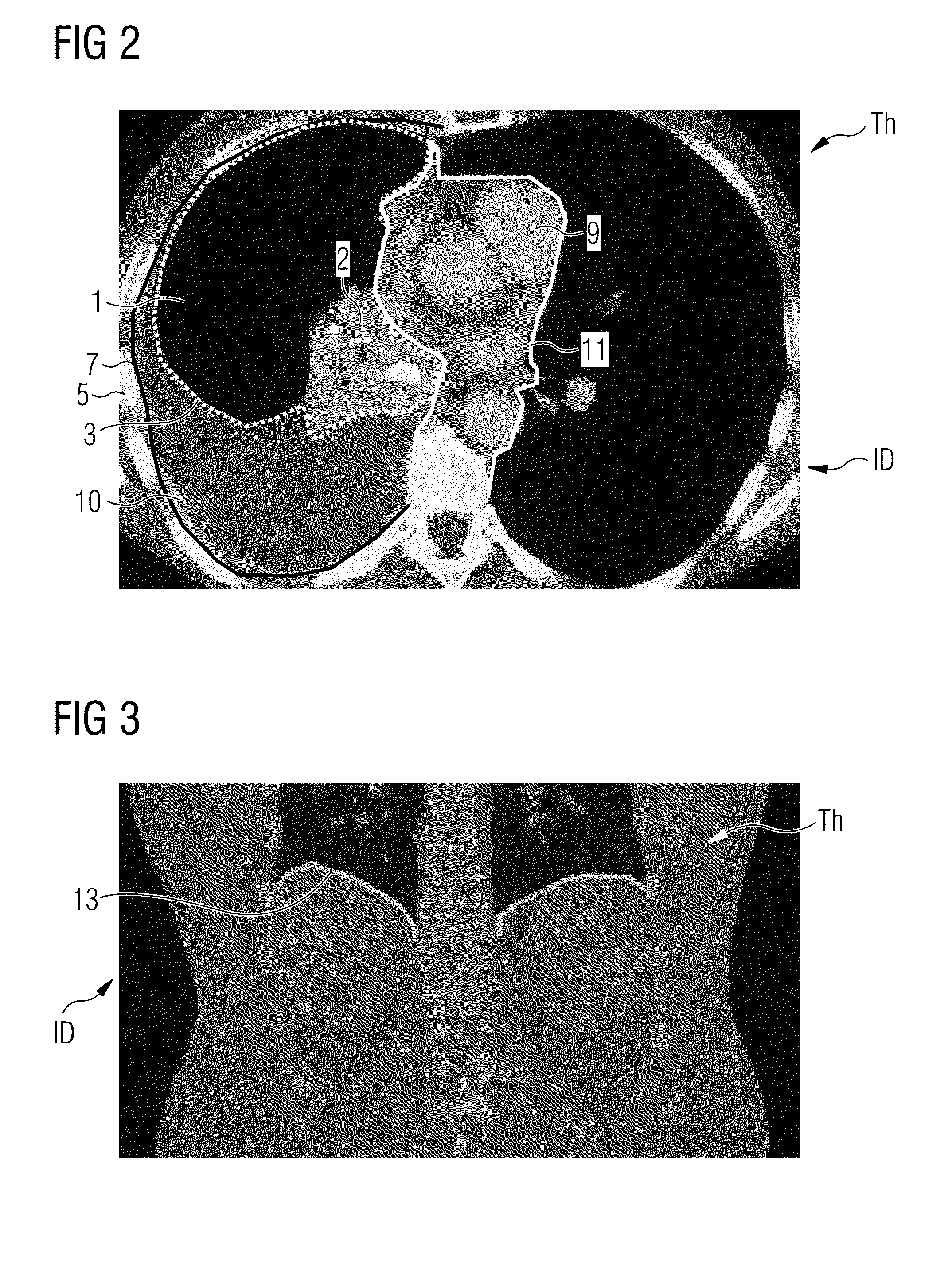
Automatic identification of a potential pleural effusion
ActiveUS20150297164A1Easy to calculatePrevent discomfort and even complicationUltrasonic/sonic/infrasonic diagnosticsImage enhancementChest regionThoracic cavity
A method for automatically identifying a potential pleural effusion in medical image data of a thorax of a patient from a scan by means of a medical scanner is provided. It includes at least the steps of accepting rib cage detection data of the rib cage of the patient from the image data, which rib cage detection data include rib cage extent data of a rib cage extent of the interior of the rib cage, accepting lung detection data of the lung of the patient from the image data, which lung detection data comprise lung extent data of a lung extent of the external boundary of the lung, accepting mediastinum detection data of all organs of the mediastinum in the thorax (Th) of the patient from the image data, which mediastinum detection data comprise mediastinum extent data of a mediastinum extent of the external boundary of the mediastinum, and subtracting the lung extent and the mediastinum extent from the rib cage extent while forming pleural effusion identification data.
Owner:SIEMENS HEALTHCARE GMBH
Improved lung node puncture positioning needle assembly
PendingCN108451599AAvoid retracting into the chest wallEliminate the possibility of sliding into the mediastinumSurgical needlesTrocarMediastinum
The invention discloses an improved lung node puncture positioning needle assembly which comprises a puncture needle with an inner hole and a positioning guiding wire penetrating through the punctureneedle, a V-shaped barb is arranged at the positioning end of the positioning guiding wire, the end, opposite to the positioning end, of the positioning guiding wire is a push end, and a reinforcing part is arranged at the portion, close to the positioning end, of the positioning guiding wire. The assembly is characterized in that a bent part with the memorizing function is arranged at the portion, close to the positioning end, of the positioning guiding wire, the bent part deforms to be linear when the positioning guiding wire is inserted into the inner hole of the puncture needle, and the bent part recovers to be bent after the positioning guiding wire gets away from the inner hole of the puncture needle. When the V-shaped barb of the positioning end of the positioning guiding wire hooksthe focus of the disease, and after the puncture needle is pulled out, the V-shaped barb on the positioning guiding wire is matched with the bent part, the positioning guiding wire is stopped from moving forwards and backwards, and the probability that the positioning guiding wire is disengaged from the focus of the disease to be retracted into the chest wall or slide into the mediastinum is eradicated.
Owner:SHANGHAI PULMONARY HOSPITAL
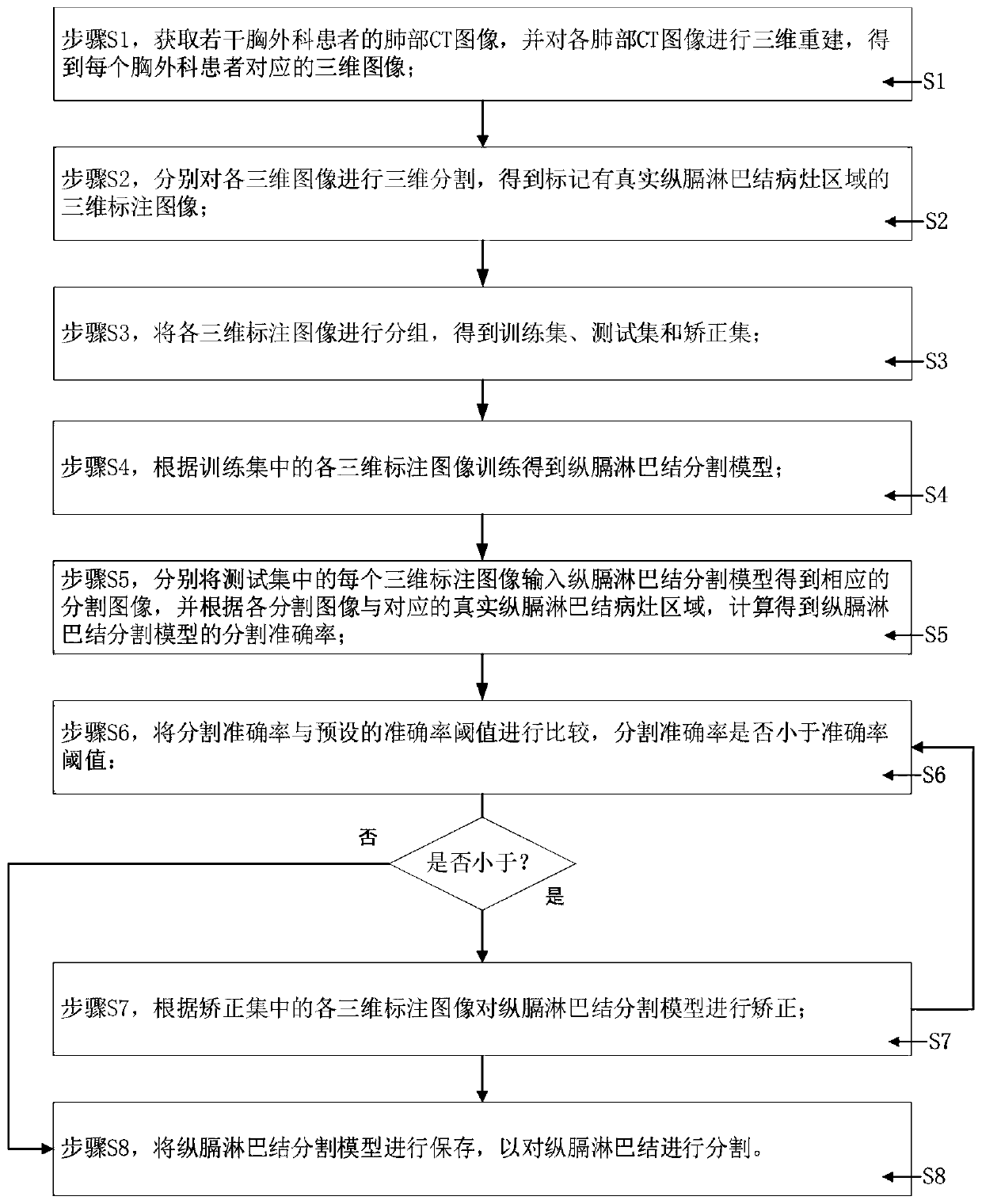
Method and system for generating diaphragm lymph node segmentation model
ActiveCN111340825AImprove accuracyImprove featuresImage enhancementImage analysisImaging processing3d segmentation
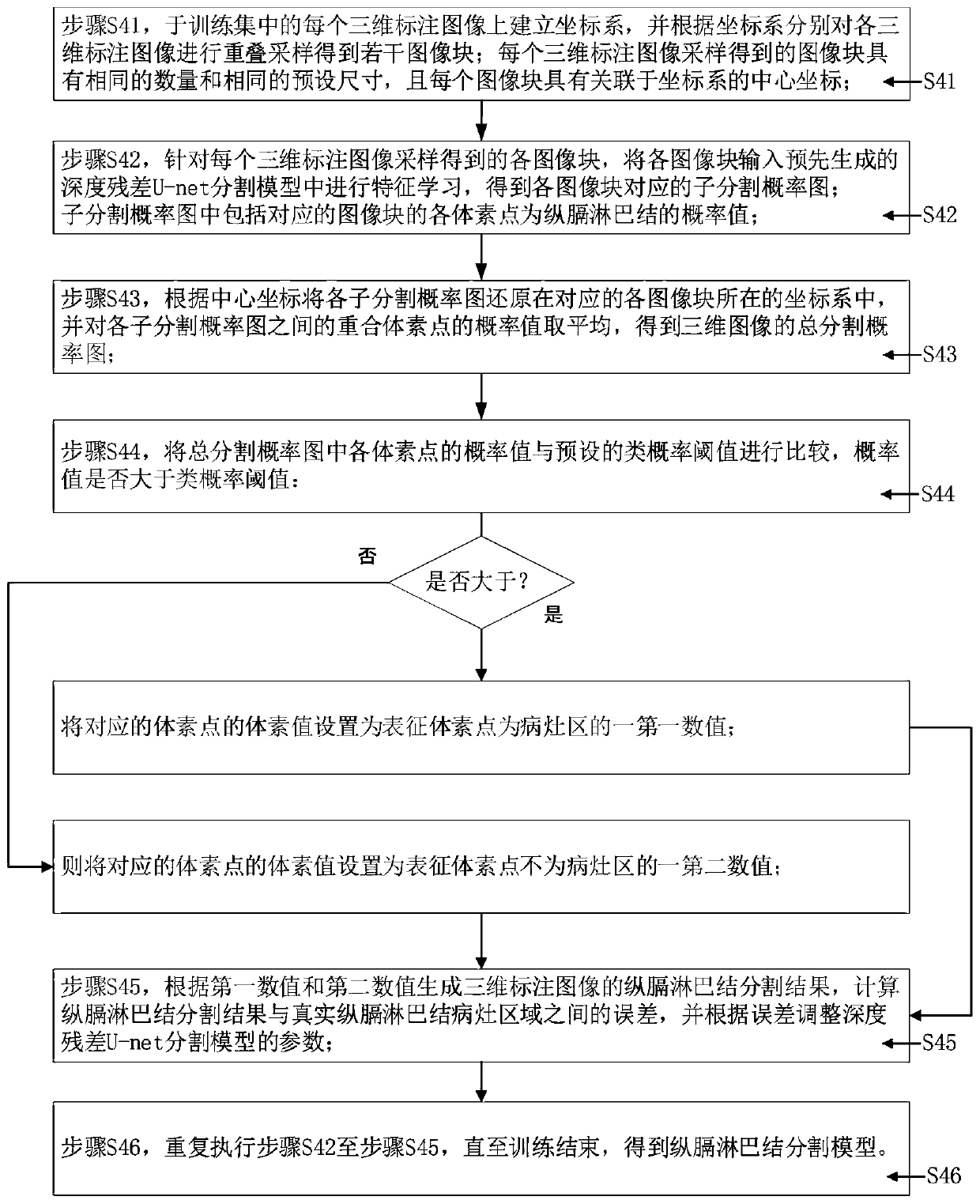
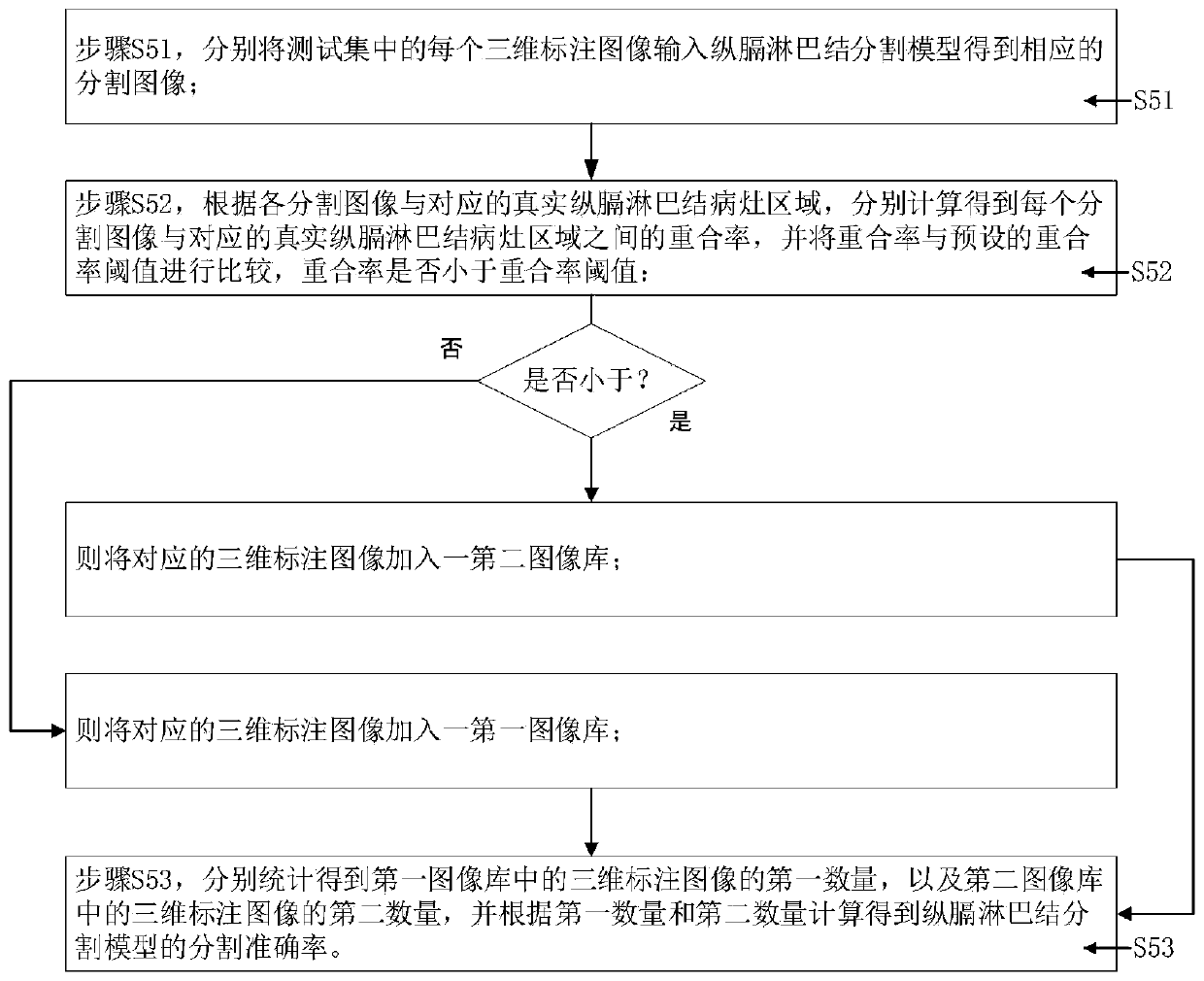
The invention provides a method and system for generating a longitudinal lymph node segmentation model, and relates to the technical field of medical image processing, and the method comprises the steps: obtaining lung CT images of a plurality of thoracic surgery patients, carrying out the three-dimensional reconstruction of each lung CT image, and obtaining a three-dimensional image; respectivelycarrying out three-dimensional segmentation on each three-dimensional image to obtain a three-dimensional marked image marked with a real longitudinal lymph node lesion area; grouping the three-dimensional annotation images to obtain a training set, a test set and a correction set; training the training set to obtain a diaphragm lymph node segmentation model; inputting the test set into a longitudinal lymph node segmentation model to obtain a corresponding segmentation image, and calculating the segmentation accuracy of the longitudinal lymph node segmentation model; if the segmentation accuracy is smaller than an accuracy threshold, enabling the correction set to correct the diaphragm lymph node segmentation model; and if the segmentation accuracy is not less than the accuracy threshold,storing the diaphragm lymph node segmentation model. The accuracy of longitudinal lymph node segmentation is effectively improved, manual intervention is not needed, and the practicability is high.
Owner:SHANGHAI PULMONARY HOSPITAL
System and method for automatic, non-invasive diagnosis of pulmonary hypertension and measurement of mean pulmonary arterial pressure
InactiveUS8301224B2Excessive analysisOptimize workflowMagnetic measurementsEvaluation of blood vesselsSystoleCardiac cycle
Owner:SIEMENS HEALTHCARE GMBH
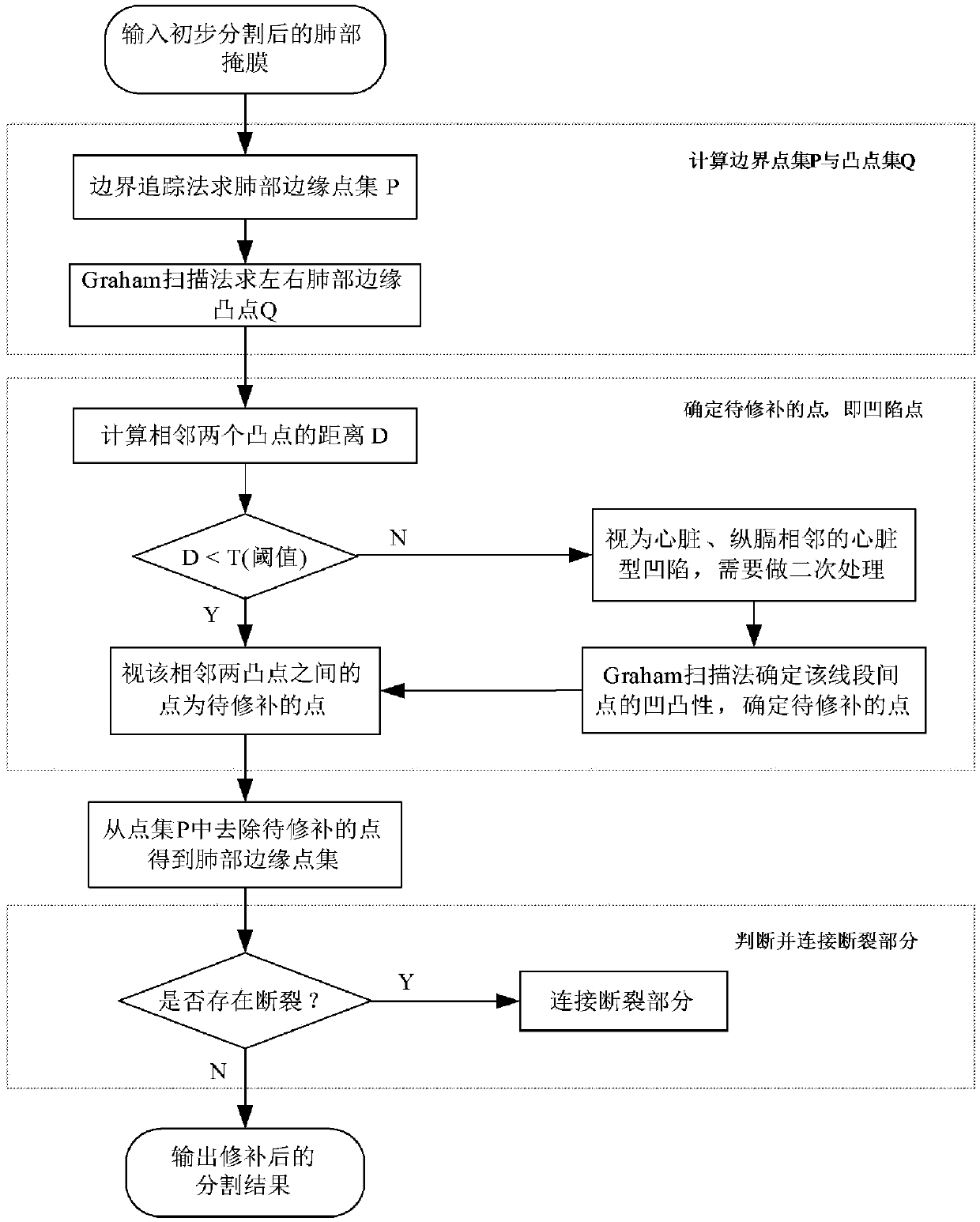
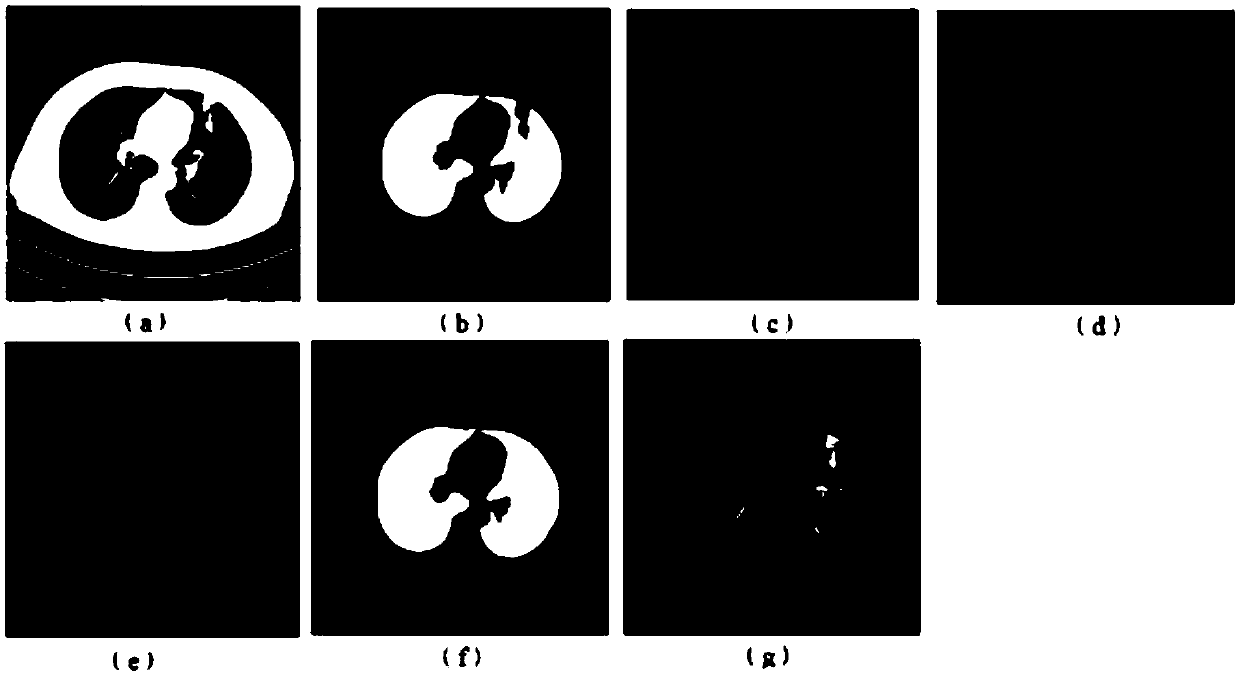
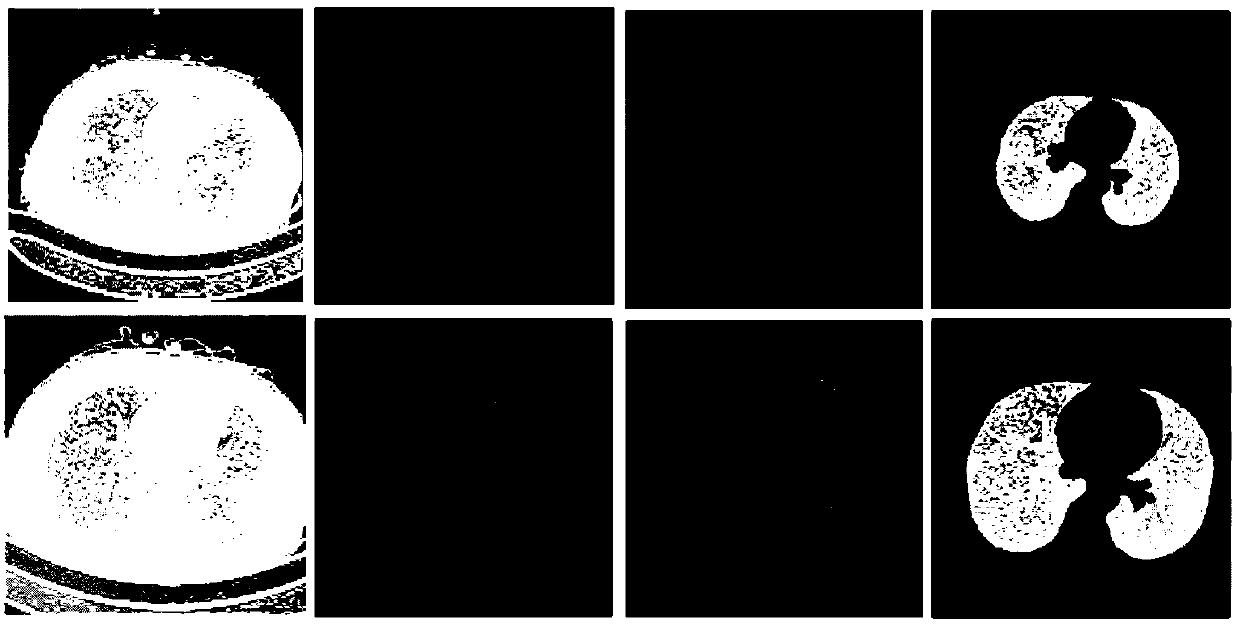
Image sag repairing method based on double-Graham scanning method
PendingCN108335277AProof of accuracyProof of validityImage enhancementImage analysisPulmonary parenchymaLeft lung
The invention relates to an image sag repairing method based on a double-Graham scanning method. The method comprises the following steps that: utilizing a boundary tracing method for a lung mask subjected to preliminary segmentation to solve the boundary point set P of a left lung and a right lung; utilizing the Graham scanning method to obtain the salient point set Q of the left lung and the right lung; solving the lung sag point to be repaired; removing the solved lung sag point to be repaired from the boundary point set P, wherein the obtained residual point set is the edge point set of the pulmonary parenchyma; detecting whether the segmented pulmonary parenchyma contains breakage or not, and if the segmented pulmonary parenchyma contains breakage, carrying out breakage connection; and finally, obtaining an integral segmented pulmonary parenchyma segmentation result. After sag repair and breakage connection are carried out, a final integral pulmonary parenchyma segmentation resultis obtained. The sag on the outer wall of the lung can be repaired, and the method performs a good repairing and smoothening function on adjacent sags including a heart, a mediastinum and the like between two pieces of pulmonary parenchyma and can provide a correct and reliable basis for subsequent pathological study.
Owner:北京中科嘉宁科技有限公司
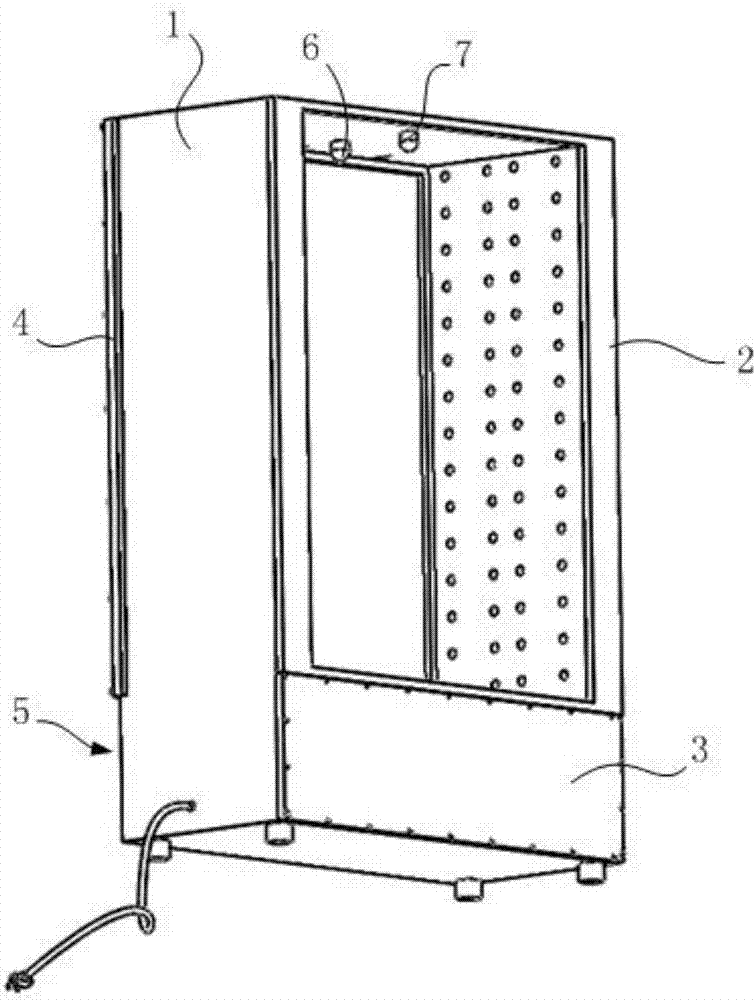
Precious archive touring cabinet
InactiveCN106942943ACompact structureLow costShow cabinetsShow shelvesControl engineeringMoisture sensor
The invention discloses a precious archives exhibition cabinet. The space of the cabinet body is divided into an upper central cavity for preventing precious archives, a lower equipment cavity, a left cavity, and a right cavity. A glass cover covers the front side of the upper central cavity. The upper rear cover covers the rear side of the upper central cavity, the front cover covers the front side of the lower equipment cavity, and the lower rear cover covers the rear side of the lower equipment cavity; the left medial partition, the right medial partition and the lower rear cover are provided with ventilation holes, and the central cavity A temperature sensor and a humidity sensor are installed on the inner side of the top wall, and an air-conditioning system is installed in the equipment chamber on the lower floor. The ventilation hole set on the left mediastinum is pumped from the upper central cavity to the left cavity and then returned to the air-conditioning system. The operation of the air-conditioning system is controlled by the signals collected by the temperature sensor and the humidity sensor. The invention has the advantages of compact structure, low cost, small weight, convenient transportation and basically no sound.
Owner:卢毅
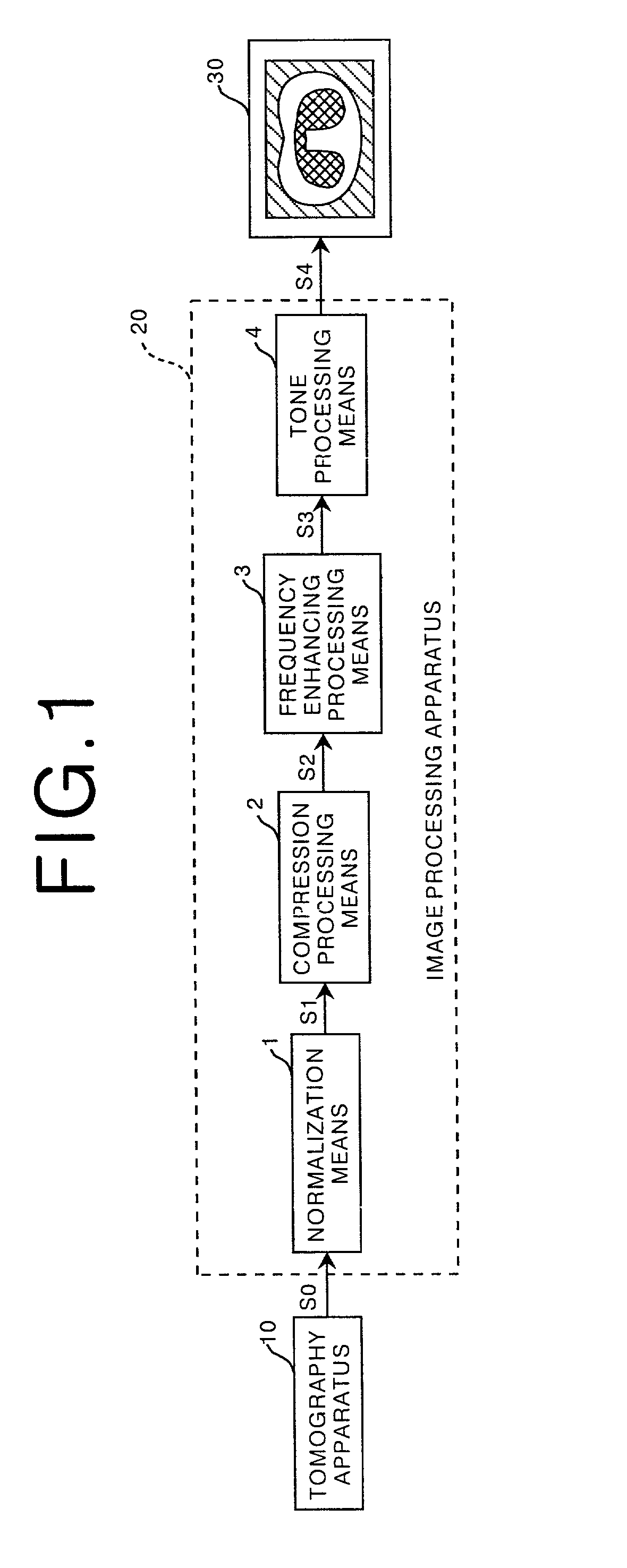
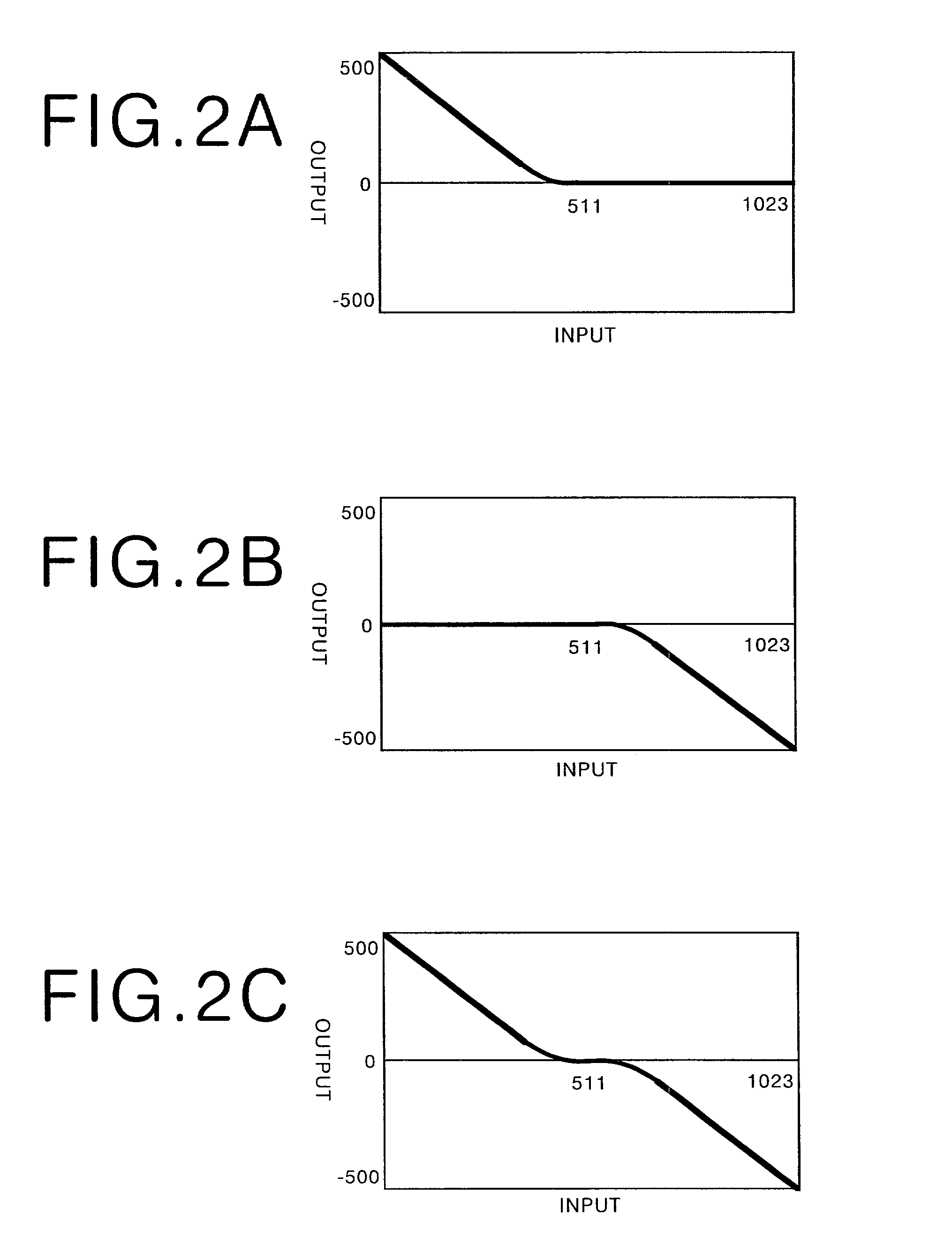
Method, apparatus, and recording medium for processing tomographic image
InactiveUS7366335B2Low densityExtended Density RangeImage enhancementImage analysisDynamic range compressionHigh density
A method and apparatus for carrying out tomographic image processing that enables lung areas and a mediastinum in a chest tomographic image to be reproduced in one image with appropriate contrast is provided. Image data representing a chest tomographic image are obtained by using a tomography apparatus. Normalization means in a tomographic image processing apparatus normalizes the image data and obtains normalized image data. Compression processing means carries out dynamic range compression processing on the normalized image data to compress a high density range thereof. The image data after the compression processing are subjected to frequency enhancing processing, and the image data after the frequency enhancing processing are then subjected to tone conversion processing. The processed image data obtained in the above manner are reproduced by a reproduction apparatus.
Owner:FUJIFILM HLDG CORP +1
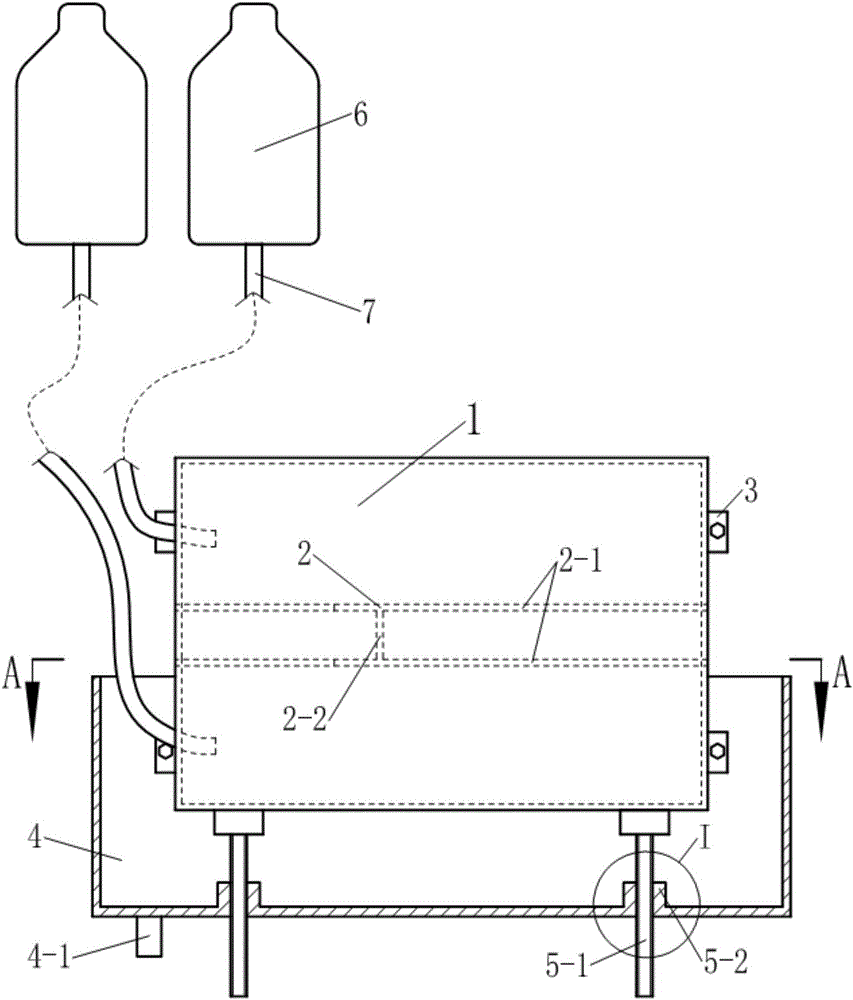
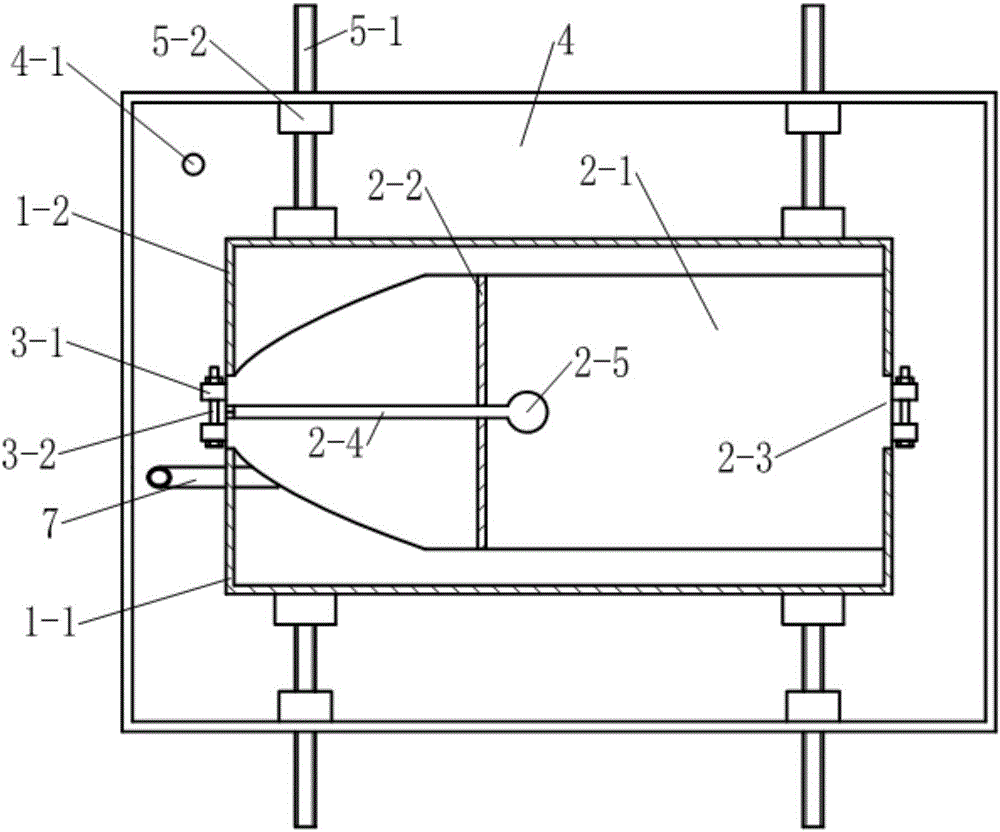
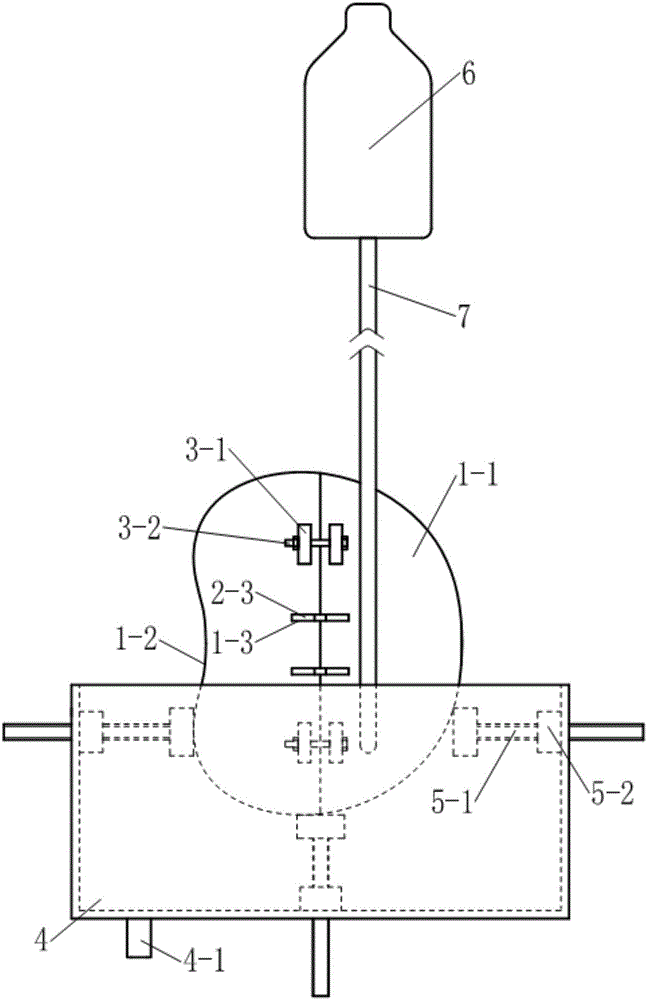
Thoracoscopic surgery simulation training box
The invention discloses a medical model, in particular, a thoracoscopic surgery simulation training box. The training box comprises a thoracic cavity prosthesis. The thoracoscopic surgery simulation training box is characterized in that the thoracoscopic surgery simulation training box further comprises a blood flow simulation device and a supporting device; the cross section of the thoracic cavity prosthesis is similar to the cross section of the thorax of a human body; the thoracic cavity prosthesis is divided into a front thoracic cavity prosthesis and a rear thoracic cavity prosthesis through a plane where midaxillary lines at two sides of the thoracic cavity prosthesis are located; a mediastinum prosthesis is arranged in the thoracic cavity prosthesis; the mediastinum prosthesis is composed of mediastinum plates located at two sides of the median sagittal plane of the thoracic cavity prosthesis and a connecting plate connected with the two mediastinum plates; the blood flow simulation device is composed of two perfusion bottles and two perfusion catheters of the thoracic cavity prosthesis; the supporting device includes a cuboid supporting box of which the top is open and supporting push rods which are located at the two sides and bottom of the supporting box; and the bottom of the supporting box is provided with a perfusion liquid recovery opening; the supporting push rod is composed of a push rod seat fixed on the box body and a push rod which passes through the push rod seat and is supported on the thoracic cavity prosthesis, wherein the push rod is in threaded connection with the push rod seat.
Owner:SOUTHERN MEDICAL UNIVERSITY
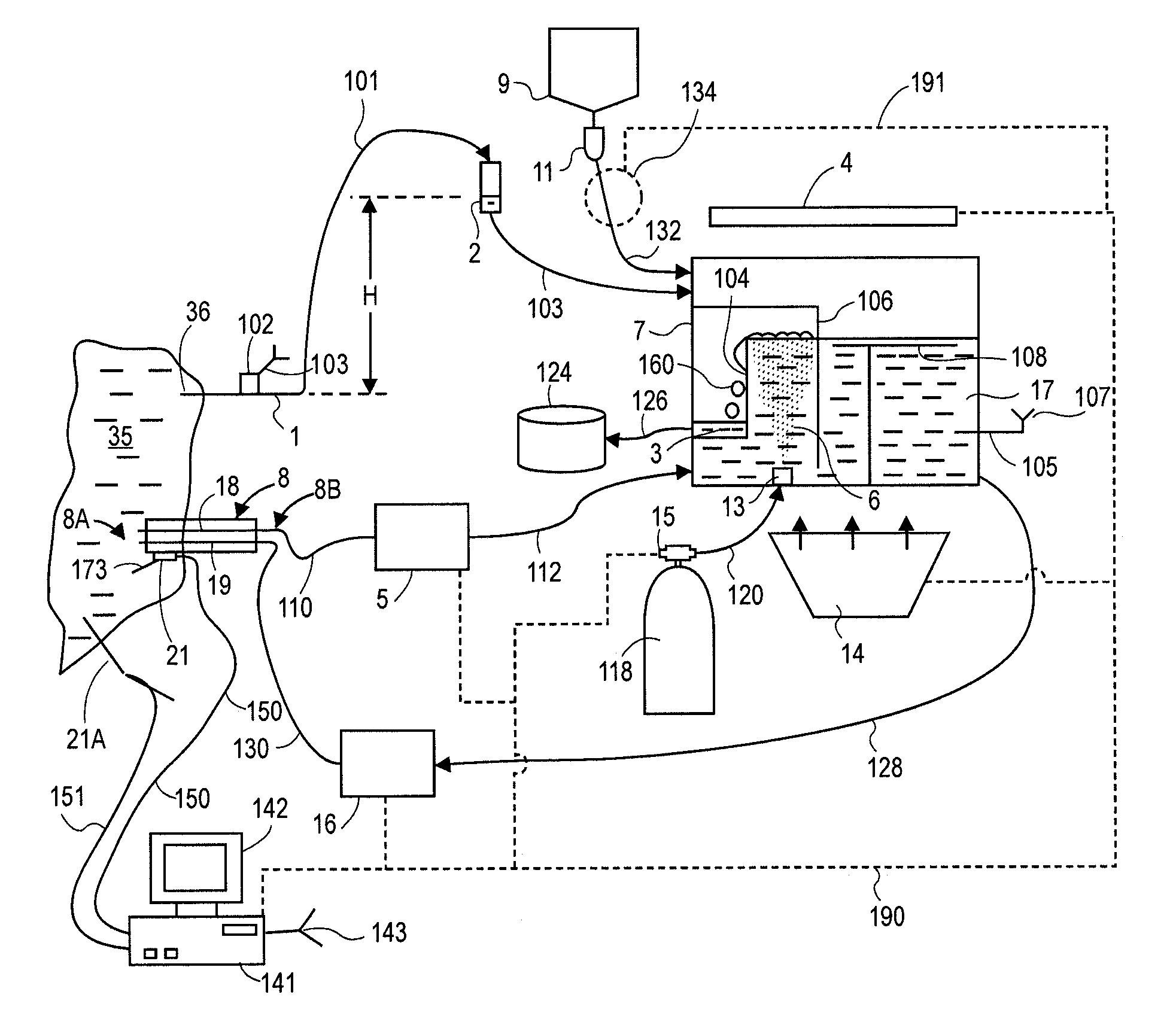
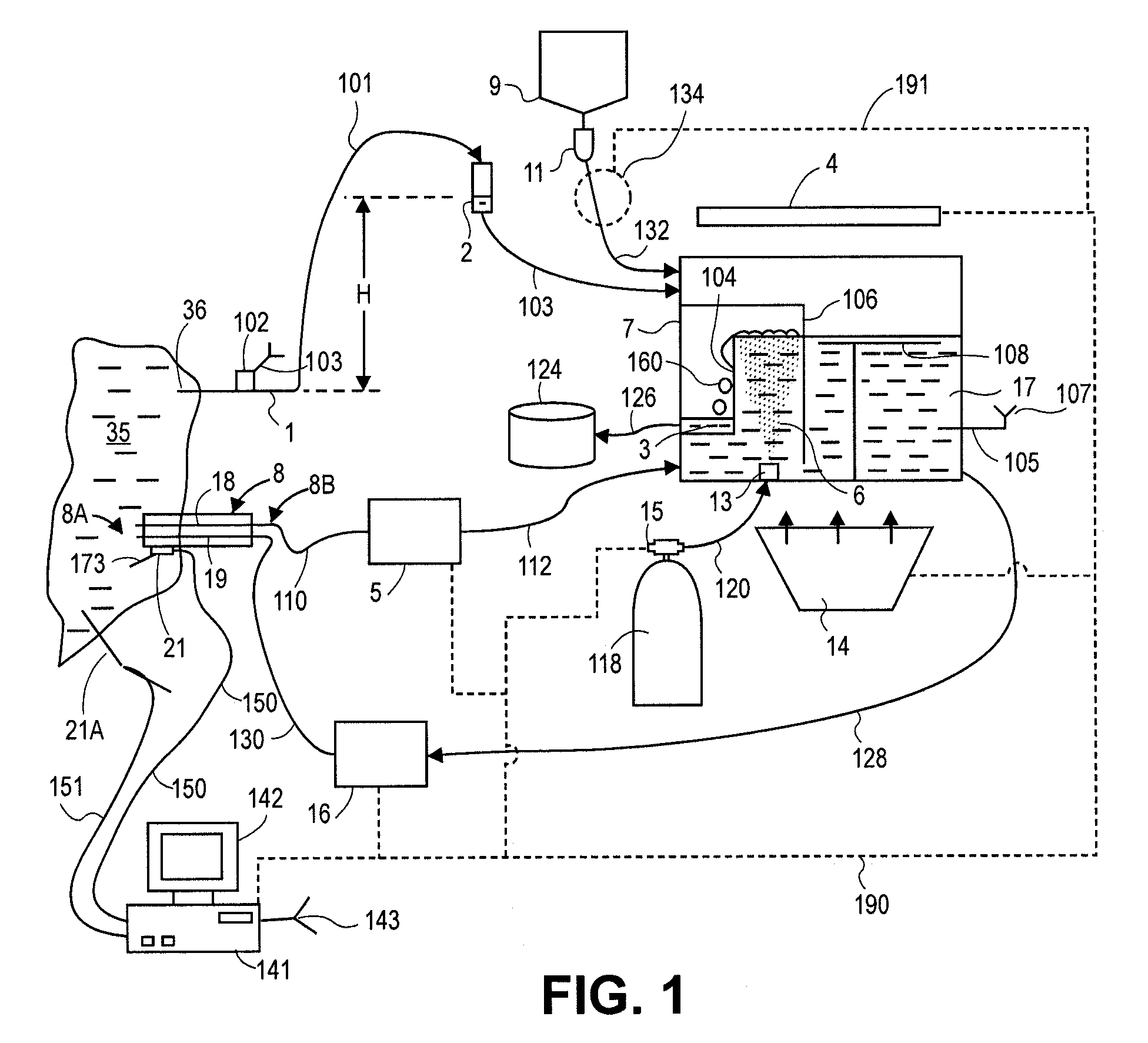
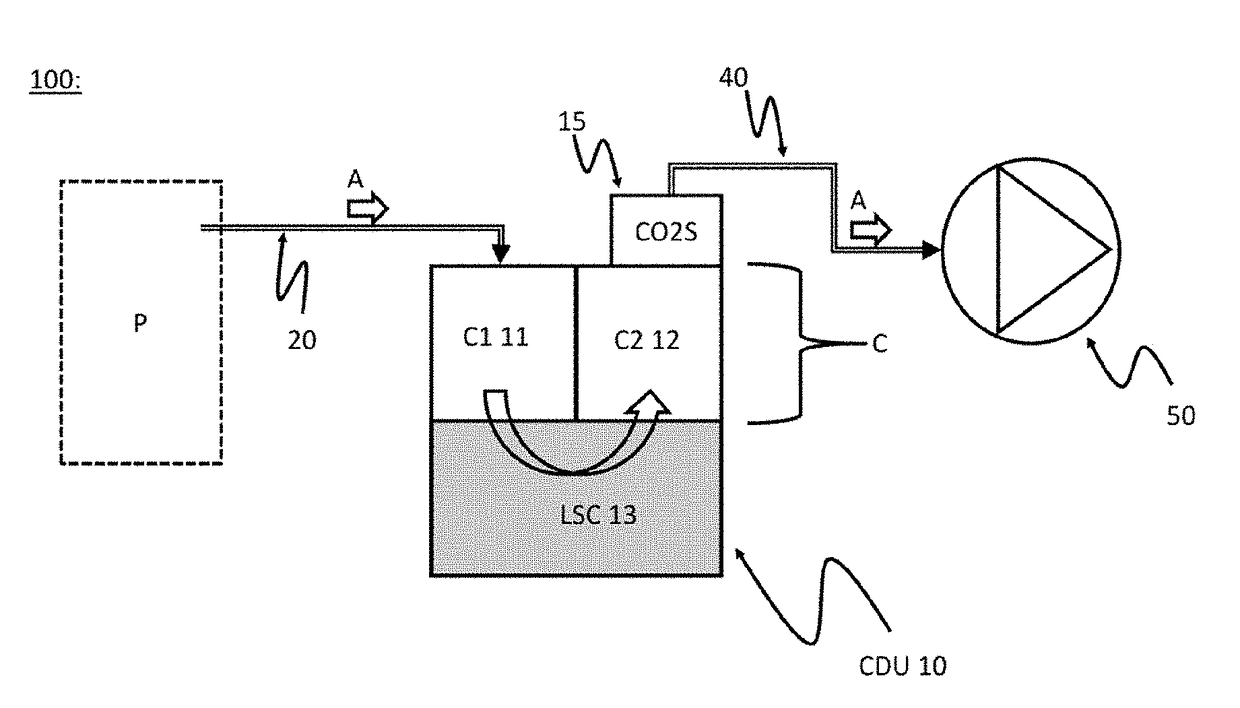
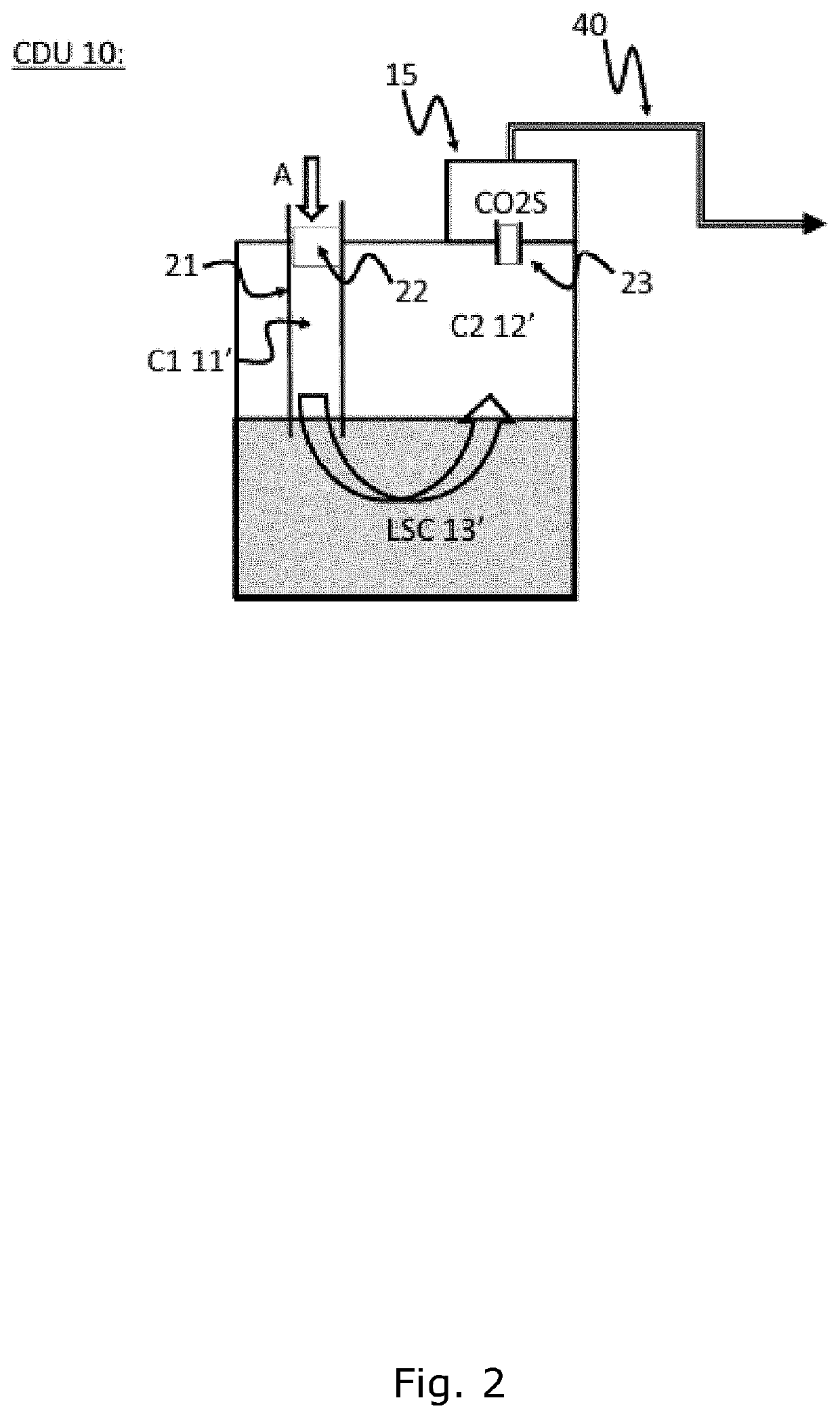
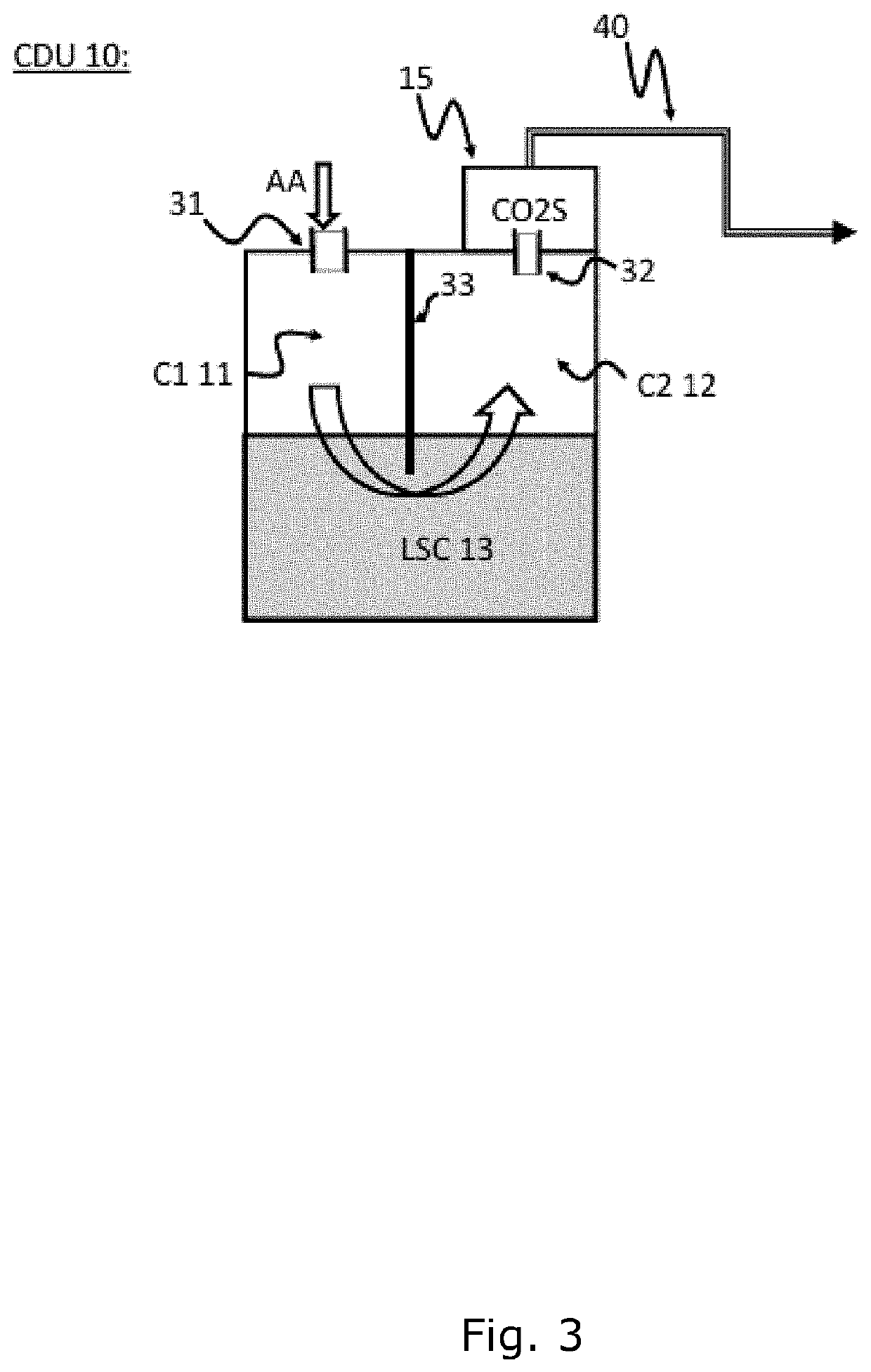
An improved chest drainage system and method
ActiveUS20180050136A1Low implementation costDirect and simple detectionMedical devicesIntravenous devicesPleural cavityChemical reaction
The invention relates to a chest drainage system (100) for creating and maintaining a sub-atmospheric pressure within the pleural cavity and / or the mediastinum of a patient (P). The system has a chest drainage unit (CDU, 10) with an internal cavity (C) having a first chamber part (C1, 11) and a second chamber part (C2, 12) with an air outlet from the CDU, and a liquid seal chamber (LSC, 13). The second chamber part (C2, 12) of the chest drainage unit is connected to a carbon dioxide sensor (CO2S, 15) for detecting carbon dioxide in any air passing through the liquid seal chamber (LSC, 13), the carbon dioxide sensor being capable of detecting carbon dioxide by a visible color change from a chemical reaction occurring in the carbon dioxide sensor between carbon dioxide and a detector reactant (DC) positioned in the carbon dioxide sensor. Preliminary test performed by the inventor have demonstrated that the present invention is very effective in determine whether, or not, carbon dioxide is present in the air passing through the chest drainage unit, this information being highly important in the subsequent decision of continuing the treatment with the chest drainage system.
Owner:REGION SYDDANMARK
Noninvasive detection kit for nasopharyngeal carcinoma susceptibility genes
The invention provides a noninvasive detection kit for nasopharyngeal carcinoma susceptibility genes. The kit comprises a specific primer, a DNA sequencing primer, a PCR (Polymerase Chain Reaction) component, a PCR product purification component, a DNA sequencing reaction component and the like, wherein the specific primer is used for detecting the polymorphic site genotypes of four mononucleotides of the Rsall polymorphism on a CYP2E1 gene, the polymorphism of a G252 site on a TNF (Tumor Necrosis Factor)-alpha gene, the deletion (Present / Null) of a GSTM (Giant Solide Turner Of The Mediastinum) 1 gene and the deletion (Present / Null) of a GSTT (Gross Saponin From Tribulus Terrestris) 1 gene. According to the kit, the risk level of nasopharyngeal carcinoma of a subject is evaluated according to the genotypes of two mononucleotide polymorphic sites closely related to the nasopharyngeal carcinoma, and then, according to the gene detection results of each subject, the subjects are guided from the gene level to specifically prevent the nasopharyngeal carcinoma so as to reduce the morbidity risk rate of the nasopharyngeal carcinoma. The sampling method of the kit adopts oral mucosal cell sampling which is painless and non-invasive, and cross-infections are avoided. The sequencing detection result of the kit is accurate and reliable, and is easy to popularize because expensive import special instruments are not needed to be purchased.
Owner:解码(上海)生物医药科技有限公司
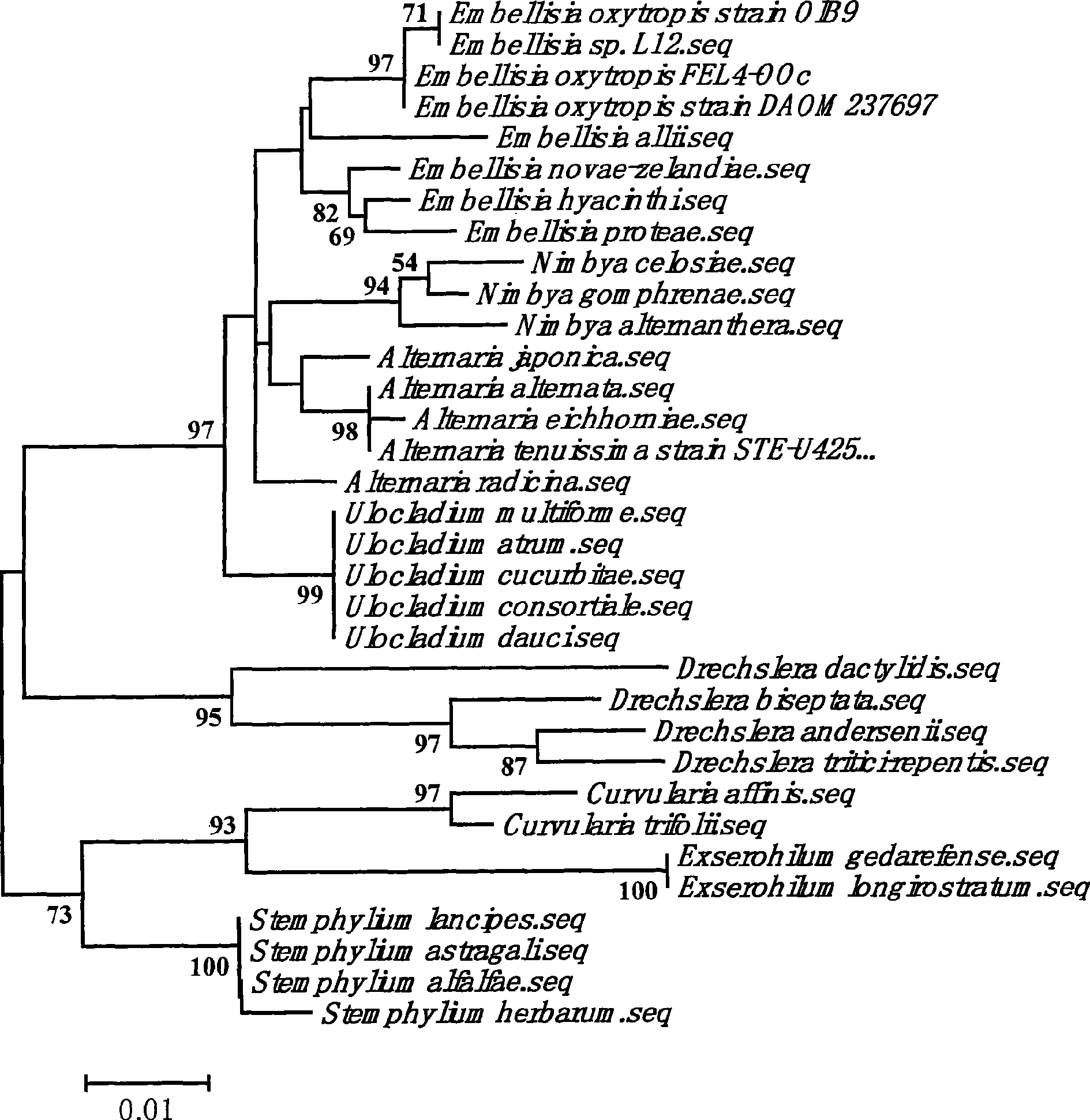
Oxytropis ehrig cinerea FEL4-OOc and separation method and application thereof
The invention discloses an oxytropis ehrig fungus alternaria FEL4-OOc which is preserved in the China type culture collection center on October 8, 2008, and the preservation code is CCTCC NO: M 208143. Bacterial colony of bacterial strain is in a circular shape with regular edges, the bacterial colony is white during early culturing period and gradually changes to gray, celadon and black as the culturing time is prolonged, and the centre of the bacterial colony slightly protrudes. Mycelium has transverse septa but has no mediastinums, and is relatively dense. Conidiophores peduncles grow on Part of the aging hypha, and the conidiophores peduncles have braches. The conidiophores are less in amount and brown in color, and are singly distributed in a scattered mode. The conidiophores have the2-5 transverse septa with thicker transverse septa films but have no mediastinums, ; spores are in long rod shapes and column shapes with the size of 30 to 90 microns*5 to 10 microns. The bacterial strain is obtained from leaves, stems, flowers or seeds of yellowflower crazyweed herbs through manual separation and filtration, and has the function of synthesizing main toxin, i.e. spherosin of the yellowflower crazyweed herbs.
Owner:NORTHWEST A & F UNIV
Oxytropis ehrig verticillium FEL5-AS1 of synthesized swainsonine and application thereof
The invention discloses an oxytropis ehrig verticillium FEL5-AS1 of a synthesized swainsonine which is preserved in China Center for Type Culture Collection on 8th, Oct of 2008, with the preservation number of CCTCC NO: M 2008144. The colony of the bacterial strain is round, which is white at first. With the extension of the culturing time, the colony gradually changes into straw yellow, tan and pitchy, and the center processes a little. Mycelium has transverse layers but has no mediastinum. Part of aged mycelia produces conidiophores which have branches. The conidiophores bend like knee bending, and spore formation scars are obvious. Conidia and conidiophores have transverse layers but have no mediastinum. The diaphragm of the conidia is thick, and the transverse layers consist 1-4. The spores are bar-shaped, similar to column. The size of the conidia is 20-90Mum multiplied by 5-10 Mum. The bacterial strain is obtained from leaves, stems and seeds of astragalus strictus by artificial separation and screening and has the function of synthesizing swainsonine (SW) which is main toxin of locoweed.
Owner:NORTHWEST A & F UNIV
Chest wall negative pressure supporting device
PendingCN112156241ACause secondary damagePain reliefNon-surgical orthopedic devicesSuction drainage systemsAspiratorChest cavity
The invention discloses a chest wall negative pressure supporting device. The chest wall negative pressure supporting device comprises a plastic supporting body used for being attached to the outer side of a chest wall in a sealed mode, wherein a flow guide groove is formed in the middle of the side surface being in contact with the outer side of the chest wall, of the plastic supporting body, anda negative pressure aspirator is arranged on the side surface away from the outer side of the chest wall, of the plastic supporting body. An air inlet of the negative pressure aspirator communicateswith the flow guide groove through a negative pressure air outlet formed in the plastic supporting body, and an exhaust port of the negative pressure aspirator communicates with an exhaust pipe. Compared with the prior art, the chest wall negative pressure supporting device has the advantages that the plastic supporting body is tightly attached to the outer side of the chest wall in a sealed mode,the plastic supporting body forms an outer bracket on the outer side of the chest wall to simulate ribs for supporting, swing of a longitudinal diaphragm is prevented, and the process of an on-site treatment operation is simple; and in addition, the chest cavity of a patient cannot be compressed, secondary injury to the patient cannot be caused, and the pain of the patient is greatly relieved.
Owner:胡迎春 +1
Oxytropis Ehrig cinerea FEL2-OG and separation method and application thereof
The invention discloses an oxytropis Ehrig cinerea FEL2-OG which is preserved in China type culture collection center on October 18, 2008, and the preservation code is CCTCC NO: M 208142. Bacterial colony of bacterial strain is in a circular shape, is white in the beginning, and gradually changes to primrose yellow, yellow brown and black brown, and the centre of the bacterial colony slightly protrudes. Mycelium has transverse septa but has no mediastinums. Conidiophores peduncles grow on part of the aging hypha h, and the conidiophores have braches. Conidiophores and the conidiophores peduncles have transverse septa but have no mediastinums, the transverse septa film of the conidiophores is thicker, and the number of the transverse septa is 1 to 3; spores are in long rod shapes and are similar to column shapes, and the size of the conidiophores is 20 to 70 microns*5 to 10 microns. The bacterial strain is obtained from leaves, stems and seeds of oxytropis glacialis through manual separation and filtration, and has the function of synthesizing main toxin, that is swainsonine (Swainsonine, SW) of locoweed.
Owner:NORTHWEST A & F UNIV
Storage device of liquid additives for papermaking
The invention discloses a storage device for liquid additives used in papermaking, which includes a packaging bag body, the packaging bag body includes a box body and a box cover, a cover plate is arranged in the box body, and a hole for the liquid auxiliary agent bottle to pass through is arranged in the center of the cover plate. The box is provided with a liquid additive bottle placement area, and the liquid additive bottle placement area is provided with a transverse septum and a longitudinal septum, and the transverse septum and the longitudinal septum intersect each other to separate the liquid additive bottle placement area into multiple In the cavity of the bottle, the longitudinal septum is provided with a first through slot, the vertical height of the first through slot is less than the height of the longitudinal septum, and the vertical height of the first through slot is equal to the vertical height of the transverse septum, The first slot is located at the upper end of the mediastinum. In this technical solution, in order to fix the liquid auxiliary agent bottles placed in the cavity and avoid collisions with each other during transportation, an accommodating hole made of stacked rings is provided at the bottom of each cavity, which can The lower end of the liquid auxiliary agent bottle is fixed.
Owner:成都鑫富汇纸业有限公司
Devices and methods for catheter-based cardiac procedures
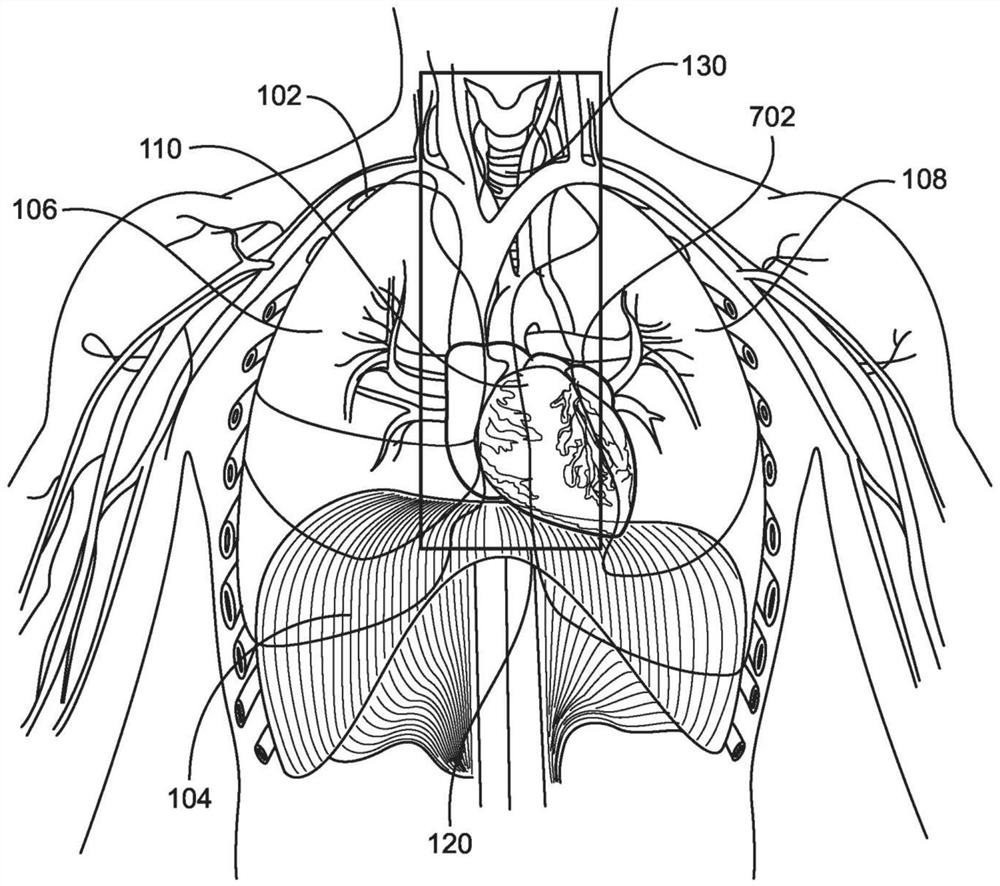
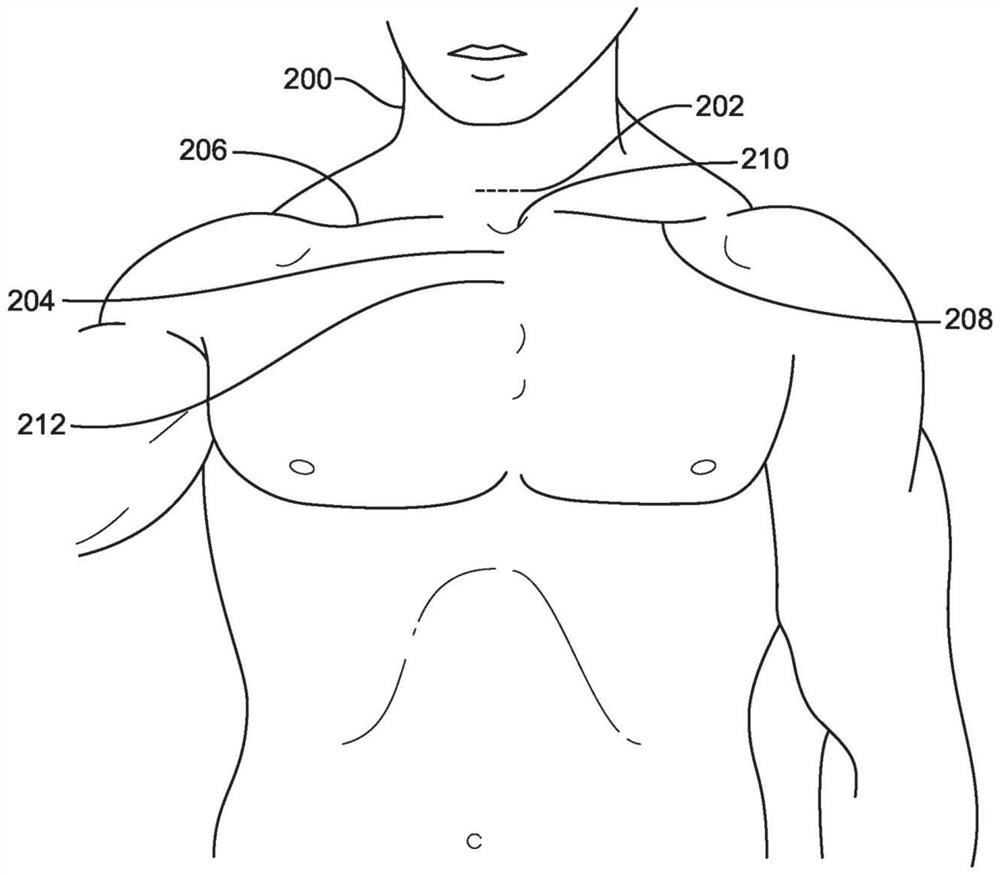
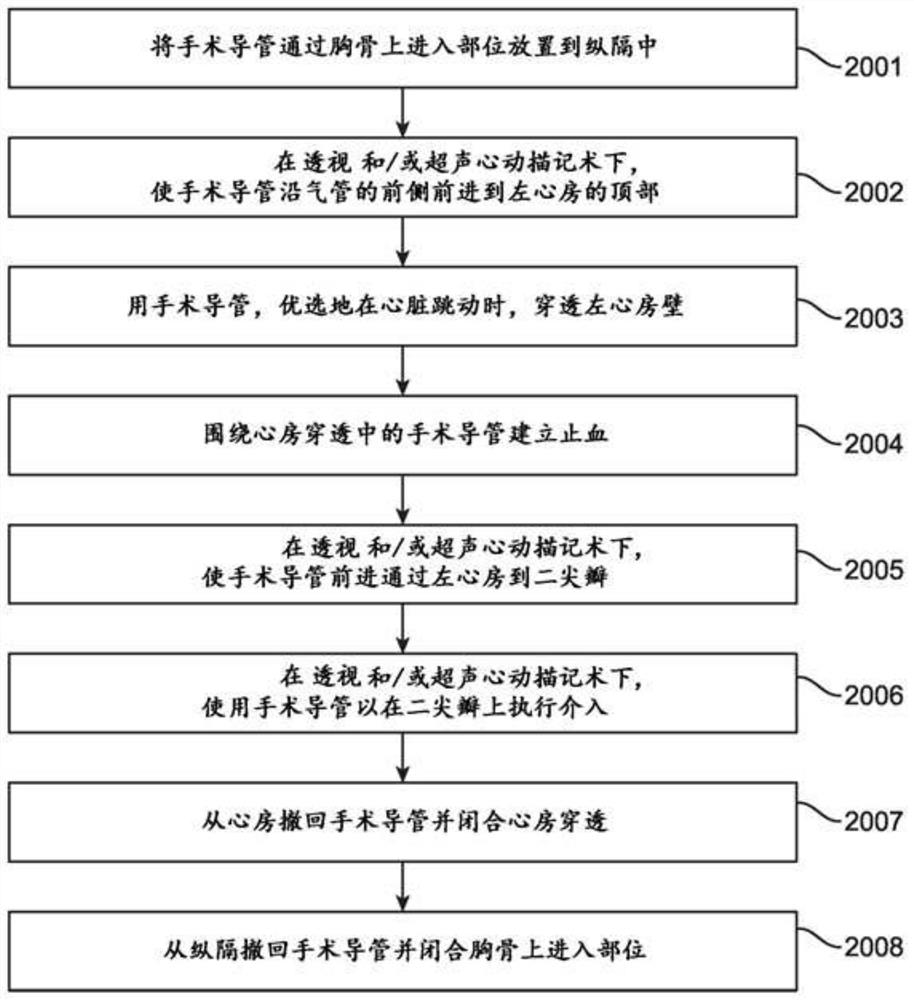
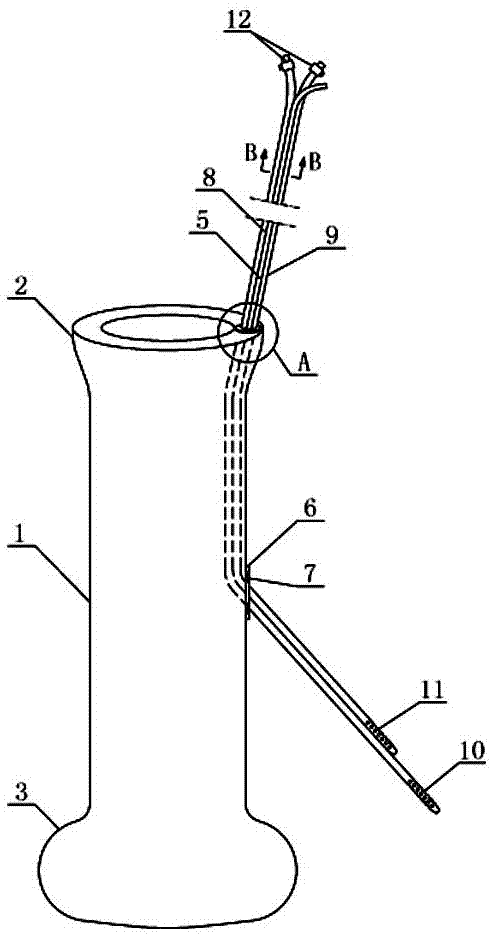
The invention provides systems, devices, and methods for performing catheter-based procedures in the heart. In specific embodiments a procedural catheter is introduced into the mediastinum from a suprasternal access site. The procedural catheter is passed through a wall of the heart, preferably at an extrapericardial location on the atrial dome. The procedural catheter is used to perform a procedure in the heart such as mitral valve repair or replacement, using remote catheter visualization techniques.
Owner:MITRX INC
Blocking drainage device for esophagogastric anastomotic leakage
PendingCN107495993AGuaranteed air tightnessWear out smoothlySurgeryMedical devicesMetal sheetTherapeutic effect
The invention discloses a blocking drainage device for esophagogastric anastomotic leakage. The blocking drainage device comprises a flexible air bag adopting a jacket structure, wherein the flexible air bag consists of an inner side wall and an outer side wall which are integrally sealed by a sealing face;the upper part and the lower part of the flexible air bag are expanded to form an upper expanded segment and a lower expanded segment; a drainage tube body penetrating the top of the upper expanded segment is arranged in the flexible air bag; the lower part of the drainage tube body outwardly penetrates the middle of the outer side wall of the flexible air bag; a first flexible sealing gasket is arranged in the position, corresponding to the drainage tube body, at the top of the upper extended part; an air inlet and outlet pipe with an air inlet and outlet valve is arranged on the first flexible sealing gasket in an upwardly extending manner; a second flexible sealing gasket is arranged in the position, corresponding to the drainage tube body, on the outer side wall of the flexible air bag; and metal sheets are embedded into the second flexible sealing gasket at intervals. The blocking drainage device has the following advantages: anastomotic leakage is blocked by the flexible air bag, so that tight blockage and high degree of comfort are realized; and cleaning treatment is performed on vomicae in the chest or the mediastinum of a patient through a flushing pipe and a draining pipe, and the treatment effect is effectively guaranteed.
Owner:吴刚
Chest drainage system and method
ActiveUS10973961B2Low implementation costDirect and simple detectionBone implantWound drainsChemical reactionPleural spaces
Owner:REGION SYDDANMARK
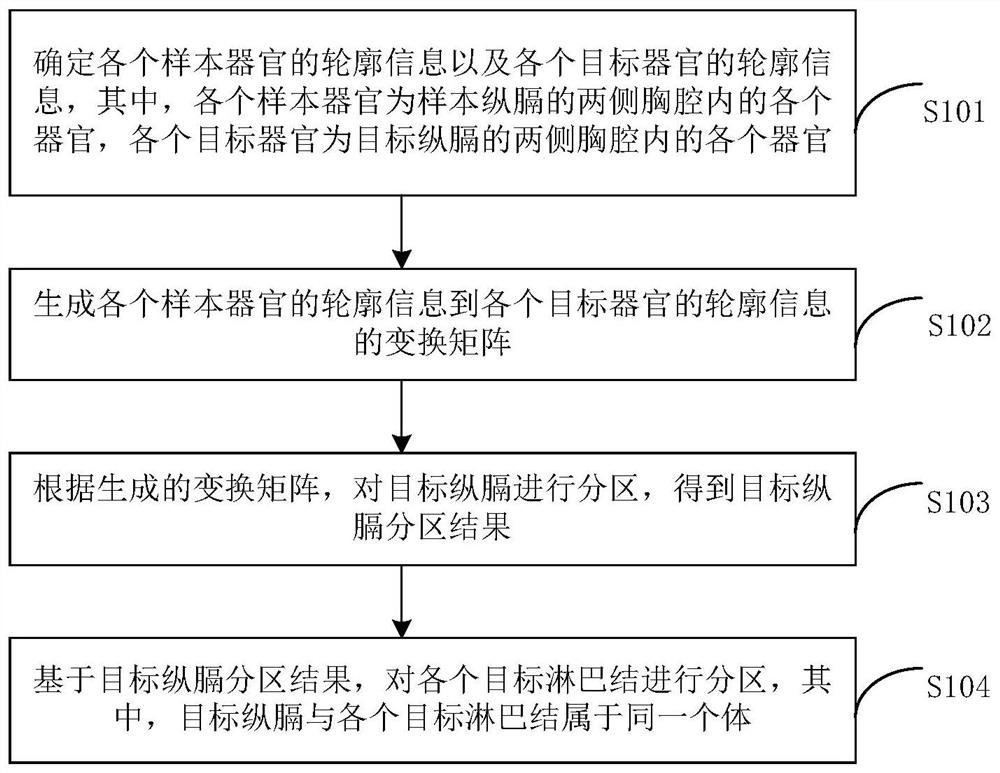
Lymph node partitioning method, device and equipment and computer readable storage medium
ActiveCN112258499AAvoid the disadvantages of manual partitioningImprove accuracyImage enhancementImage analysisThoracic structureThoracic cavity
The invention provides a lymph node partitioning method which comprises the following steps: determining contour information of each sample organ and contour information of each target organ, each sample organ being each organ in thoracic cavities at two sides of a longitudinal diaphragm of a sample, and each target organ being each organ in thoracic cavities at two sides of a target longitudinaldiaphragm; generating a transformation matrix from the contour information of each sample organ to the contour information of each target organ; partitioning the target longitudinal diaphragm according to the transformation matrix to obtain a target longitudinal diaphragm partitioning result; and partitioning each target lymph node based on the target longitudinal partition result. Visibly, each target lymph node can be automatically partitioned, the defects of manual partitioning are avoided, and the accuracy of lymph node partitioning results is improved.
Owner:BEIJING SHENRUI BOLIAN TECH CO LTD +1
Oxytropis Ehrig cinerea FEL2-OG and separation method and application thereof
The invention discloses an oxytropis Ehrig cinerea FEL2-OG which is preserved in China type culture collection center on October 18, 2008, and the preservation code is CCTCC NO: M 208142. Bacterial colony of bacterial strain is in a circular shape, is white in the beginning, and gradually changes to primrose yellow, yellow brown and black brown, and the centre of the bacterial colony slightly protrudes. Mycelium has transverse septa but has no mediastinums. Conidiophores peduncles grow on part of the aging hypha h, and the conidiophores have braches. Conidiophores and the conidiophores peduncles have transverse septa but have no mediastinums, the transverse septa film of the conidiophores is thicker, and the number of the transverse septa is 1 to 3; spores are in long rod shapes and are similar to column shapes, and the size of the conidiophores is 20 to 70 microns*5 to 10 microns. The bacterial strain is obtained from leaves, stems and seeds of oxytropis glacialis through manual separation and filtration, and has the function of synthesizing main toxin, that is swainsonine (Swainsonine, SW) of locoweed.
Owner:NORTHWEST A & F UNIV
Pericardium mediastinum drainage device capable of continuously generating negative pressure
PendingCN111467587AIncrease in sizeThere will be no situation of being ripped outMedical devicesIntravenous devicesCatheterPericardium
The present invention discloses a pericardium mediastinum drainage device capable of continuously generating negative pressure. The pericardium mediastinum drainage device capable of continuously generating negative pressure comprises a balloon (1), a head end of the balloon (1) is provided with a one-way valve I (2), and the one-way valve I (2) is connected with a pericardium mediastinum drainagetube (3); a tail end of the balloon (1) is provided with a one-way valve II (4) and the one-way valve II (4) is connected with a catheter (5); and a flow direction of the one-way valve I (2) is the same as a drainage direction and the flow directions of the one-way valve I and the one-way valve II are the same. The pericardium mediastinum drainage device has characteristics of convenient operation and high safety.
Owner:GUIZHOU PROVINCIAL PEOPLES HOSPITAL
Splicing type double-balloon contrast agent conveying pipe for salpingography
InactiveCN114849037AEasy injectionLess discomfortBalloon catheterMulti-lumen catheterCatheterMediastinum
The invention discloses a spliced double-balloon contrast agent delivery tube for salpingography, which comprises a delivery catheter connected to the inner side of a breather tube in a penetrating manner, a balloon tube nested on the outer side of the breather tube, and a drug delivery three-way catheter fixedly mounted at the rear end of the delivery catheter, the upper end of the conveying catheter is provided with a medicine inlet valve, the gas injection three-way catheter is fixedly installed at the rear end of the breather pipe, the upper end of the gas injection three-way catheter is provided with a gas injection sealing plug and a medicine injector, and the gas injection three-way catheter is installed on the rear side of the medicine conveying three-way catheter in a communicating mode. The spliced double-balloon contrast agent delivery tube for salpingography can be provided with two balloon structures at the same time, and contrast agents can be injected from the double delivery tubes at the same time, so that contrast observation can be conveniently carried out on uterine cavities of patients with double uteruses or complete mediastine uterus at the same time, discomfort of the patients is reduced, and the working efficiency of the patients is improved. And the radiography efficiency and quality are effectively improved.
Owner:QINGDAO MUNICIPAL HOSPITAL
Integrated xiphoid costal margin lower orientating and positioning guider and using method thereof
InactiveCN105615958AEnsure safetyAvoid accidental damageSurgical needlesCatheterEngineeringEndoscope
The invention discloses an integrated xiphoid costal margin lower orientating and positioning guider and a using method thereof. The integrated xiphoid costal margin lower orientating and positioning guider comprises a first guiding pipe and a second guiding pipe which are arranged in a coaxial and separated mode, and the first guiding pipe and the second guiding pipe are fixedly connected through a connecting rod; the first guiding pipe is of a pipe-shaped structure of which the interior is provided with a cylindrical cavity in a communicated mode, a hollow semicircular pipe with an upward opening is adopted as the second guiding pipe, and a guiding channel allowing a puncturing device to penetrate into is formed in the cylindrical cavity and the hollow semicircular pipe. Accordingly, the problem that in a xiphoid lower anterior mediastinum operation through a novel endoscope, no appropriate instrument for xiphoid lower gap separating and guiding exists is solved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
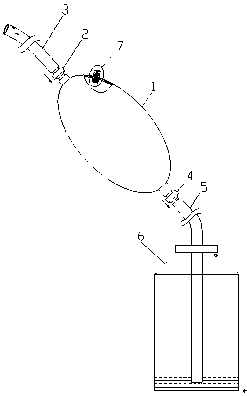
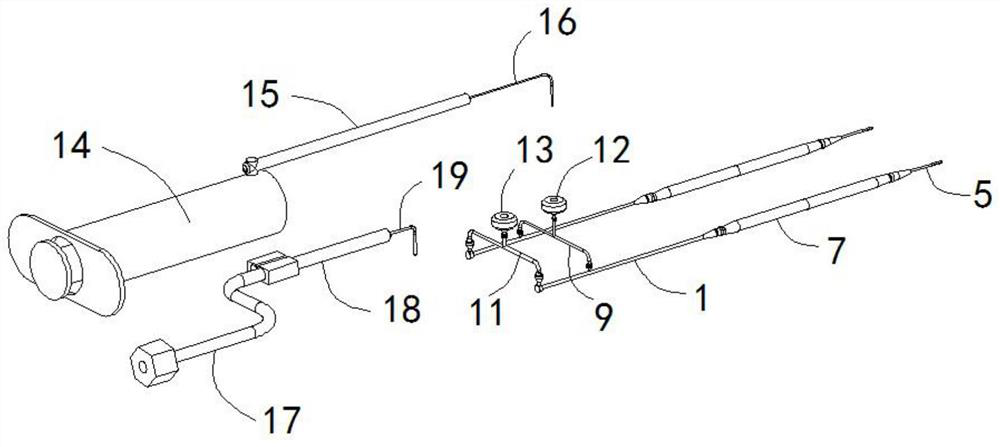
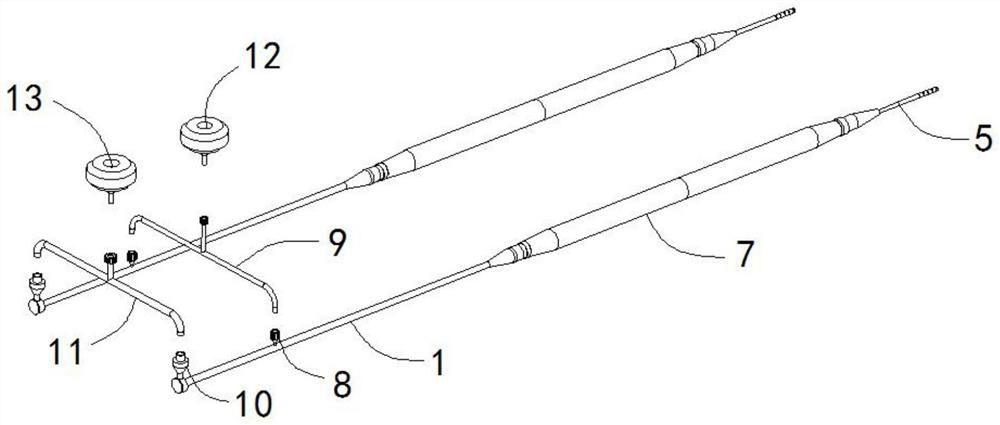
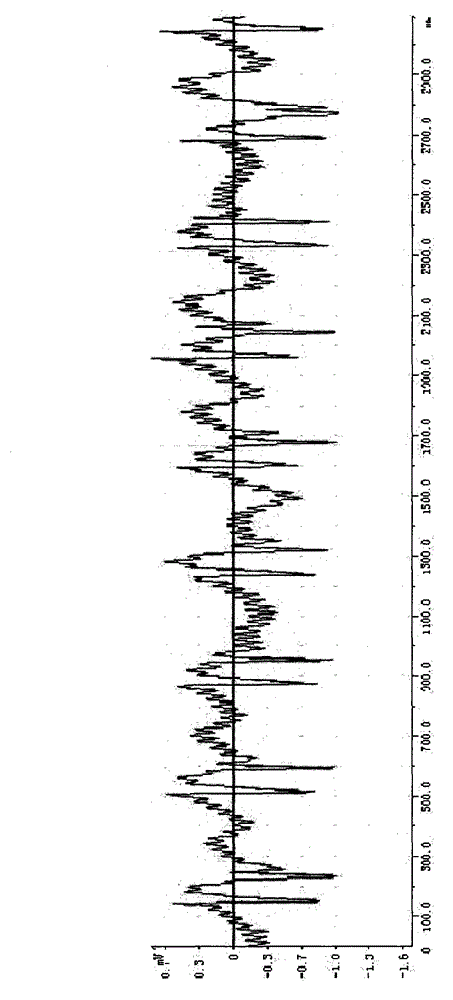
Establishing method for rabbit sinus node chronic injury model and special electrode for implementing establishing method
The invention provides an establishing method for a rabbit sinus node chronic injury model. The method comprises the following steps of: after anaesthetizing a rabbit, fixing the back of the rabbit on a rabbit table, and recording body surface electrocardiogram; shaving the skin at a colpus area, longitudinally opening the chest along a third rib at the right side of sternum, separating epidermis and muscular tissues layer by layer, cutting off the third rib of the right side, and opening pericardium through mediastinum to expose auricula dextra; placing an electrode connected with an electrocardiogram V1 lead to a near sinus node area of the rabbit and slowly moving, when the electrocardiogram displays electrograph characteristics of the sinus node area, applying 20% formaldehyde solution to the electrode area, when the heart rate of the rabbit is decreased to 50-70% of original heart rate, intravenously injecting 2mg of atropine sulphate until the heart rate stops rising, and stopping applying formaldehyde solution; and cleaning the chest, closing the chest layer-by-layer, and feeding for later use. The invention also provides a sinus node targeted injection penetration electrode for implementing the method. Compared with the prior art, the establishing method is high in modeling success rate, stable in model and good in feasibility and repeatability..
Owner:GUANGANMEN HOSPITAL CHINA ACAD OF CHINESE MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com