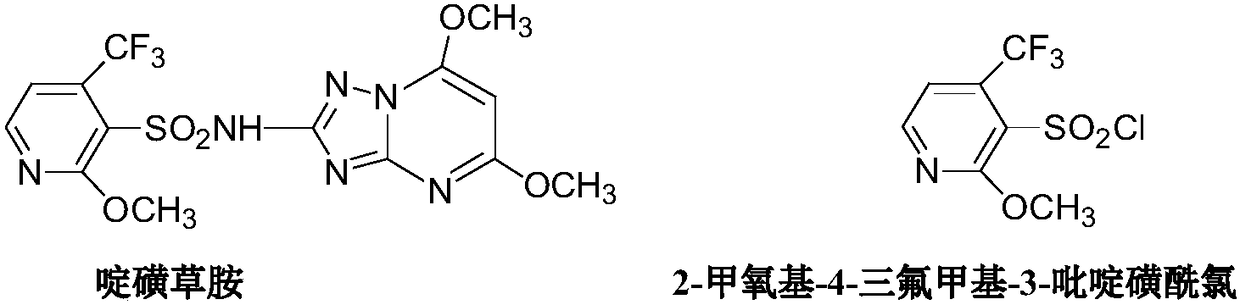
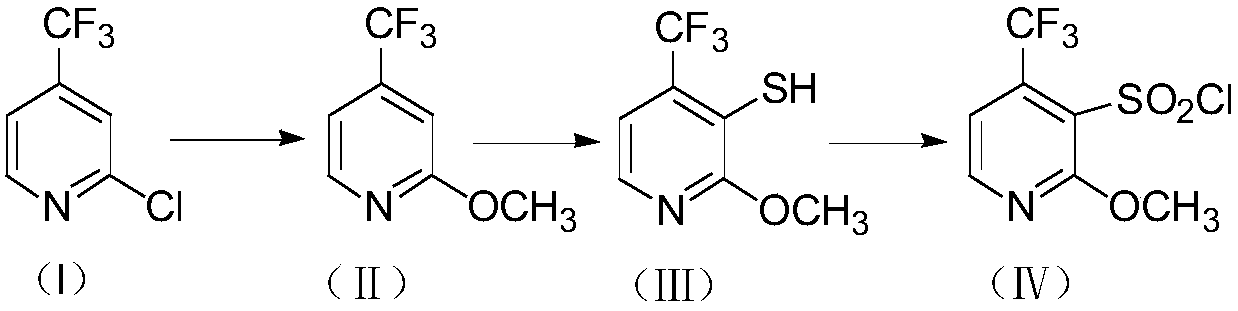
Method for preparing 2-methoxy-4-trifluoromethyl-3-pyridinesulfonyl chloride
A technology of trifluoromethylpyridine and pyridinesulfonyl chloride, which is applied in the direction of organic chemistry, can solve the problems of unfavorable environmental protection, low safety factor, ether solution anesthesia, etc., and achieves the advantages of production safety, environmental protection and odor reduction The effect of sexual odor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of compound Ⅱ
[0033]
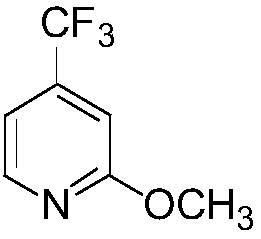
[0034] Add 2-chloro-4-trifluoromethylpyridine (10.0g, 0.055mol) into the reaction flask, and 10% sodium methoxide solution (30.0g, 0.055mmol) with a mass fraction of 10%, heat up and react at 55°C, GC tracked that the reaction of the raw materials was complete. After the reaction was completed, cool to room temperature, filter, recover methanol in vacuo, add water and dichloromethane to extract and separate layers, and concentrate the dichloromethane under reduced pressure to obtain 8.38 g of light yellow liquid with a yield of 86.1%. 1 H-NMR (300MHZ, CDCl 3 )δ8.23(d, J=3HZ, 1H, pyridine ring hydrogen), 6.99(d, J=6HZ, 1H, pyridine ring hydrogen), 6.88(s, 1H, pyridine ring hydrogen), 3.89(s, 3H, -OCH 3 ).
[0035] Preparation of compound Ⅲ
[0036]
[0037] Under nitrogen protection, add n-hexane (140ml), diisopropylamine (5.1g, 0.05mol) into the reaction flask, cool to -60°C and add dropwise 2.2M n-butyllithium cyclohexane ...
Embodiment 2
[0043] Preparation of Compound II:
[0044]
[0045] The raw material 2-chloro-4-trifluoromethylpyridine (18.5g, 0.102mol) was first added to the reaction flask, and 15% sodium methoxide solution (73.4g, 0.204mol) was slowly added, and after mixing, the temperature was raised to 60°C Reaction, GC tracking and analysis of raw materials After the reaction is complete, cool down to room temperature, obtain the filtrate by suction filtration, remove methanol under reduced pressure, add water and dichloromethane to extract and separate layers, and concentrate dichloromethane under reduced pressure to obtain 16.1 g of light yellow liquid. 89.2%.
[0046] Preparation of compound III:
[0047]
[0048]Under the protection of argon, in the three-necked flask, first add 250ml of tetrahydrofuran, add triethylamine (12.5g, 0.124mol) into the reaction flask, and then start to cool down to -50°C, add 2.2M n-butyllithium cyclohexane Add alkane solution (77.5ml, 0.171mol) slowly, afte...
Embodiment 3
[0053] Preparation of compound Ⅱ
[0054]
[0055] Add 2-chloro-4-trifluoromethylpyridine (45.5g, 0.251mol) in the reaction flask, after the mass fraction is 30% sodium methoxide solution (135.5g, 0.753mol) and mix well, heat up, at 65 Reaction at ℃, followed by GC until the raw material reacted completely, cooled to room temperature after the reaction, filtered, recovered methanol in vacuum, added water and dichloromethane to extract and separate layers, concentrated dichloromethane under reduced pressure to obtain 40.9 g of light yellow liquid, yield 92.1 %.
[0056] Preparation of compound Ⅲ
[0057]
[0058] Under nitrogen protection, add 2-methyltetrahydrofuran (380ml), n-propylamine (12.6g, 0.213mol) into a four-neck flask, cool to -40°C and add dropwise 2.2M n-butyllithium cyclohexane solution (116.4ml, 0.256mol), stirred for 1h, added dropwise 2-methoxy-4-trifluoromethylpyridine (37.8g, 0.213mol), reacted at -40°C for 3 hours, and added sulfur powder (8.2g, 0.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com