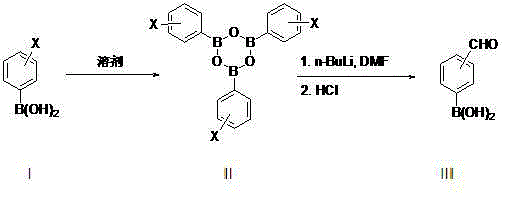
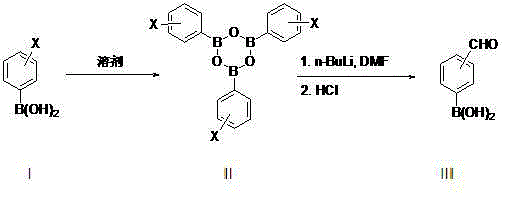
Method for preparing formyl phenylboronic acid
A technology of aldehyde phenylboronic acid and halogenated phenylboronic acid, which is applied in the field of synthesis of fine chemical intermediates, and can solve the problems of many impurities and difficult purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis of p-bromophenylboronic acid trimer (formula II, X=p-Br):
[0019] In a 1L three-necked flask equipped with a reflux water separation device, add p-bromophenylboronic acid (201 g, 1.0 mol) and 700 ml of n-heptane, heat to reflux water separation, and when the system separates about 17.5-18.5 grams of water, And when the system no longer has water to continue to separate, stop the reaction. After cooling down, the heptane was distilled to stagnant liquid to obtain the crude p-bromophenylboronic acid trimer, which contained about 2-5% heptane at this time. directly into the next reaction.
[0020] Synthesis of p-aldehydophenylboronic acid:
[0021] Under nitrogen protection, the p-bromophenylboronic acid trimer obtained above was added to 600 ml of anhydrous tetrahydrofuran to dissolve evenly, and then transferred to a 2 L three-necked flask, and then dimethylformamide (80.4 g, 1.1 mol) was added. Then lower the temperature of the system to below -70°C, and s...
Embodiment 2
[0023] Synthesis of m-bromophenylboronic acid trimer (formula II, X=m-Br):
[0024] In a 1L three-necked flask equipped with a reflux water separation device, add m-bromophenylboronic acid (201 g, 1.0 mol) and 600 ml of toluene, heat to reflux water separation, when the system separates about 17.5-18.5 grams of water, and the system The reaction was stopped when no more water continued to separate. After cooling down, the heptane was distilled to stasis to obtain the crude m-bromophenylboronic acid trimer, which contained about 5-10% toluene at this time. directly into the next reaction.
[0025] Synthesis of m-aldophenylboronic acid:
[0026] Under nitrogen protection, the m-bromophenylboronic acid trimer obtained above was added to 600 ml of anhydrous tetrahydrofuran to dissolve evenly, then transferred to a 2 L three-necked flask, and dimethylformamide (80.4 g, 1.1 mol) was added. Then lower the temperature of the system to below -70°C, and slowly add 440 ml (1.1 moles) ...
Embodiment 3
[0028] Synthesis of o-bromophenylboronic acid trimer (formula II, X=o-Br):
[0029] In a 1L three-necked flask equipped with a reflux water separation device, add o-bromophenylboronic acid (201 grams, 1.0 mol) and 600 milliliters of toluene, heat to reflux water separation, when the system separates about 17.5-18.0 grams of water, and the system The reaction was stopped when no more water continued to separate. After cooling down, the heptane was distilled to stasis to obtain the crude m-bromophenylboronic acid trimer, which contained about 5-10% toluene at this time. directly into the next reaction.
[0030] Synthesis of o-aldehyde phenylboronic acid:
[0031] Under nitrogen protection, the o-bromophenylboronic acid trimer obtained above was added to 600 ml of anhydrous tetrahydrofuran to dissolve evenly, then transferred to a 2 L three-necked flask, and then dimethylformamide (87.7 g, 1.2 mol) was added. Then cool the system down to -70°C to -80°C, and start to slowly add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com