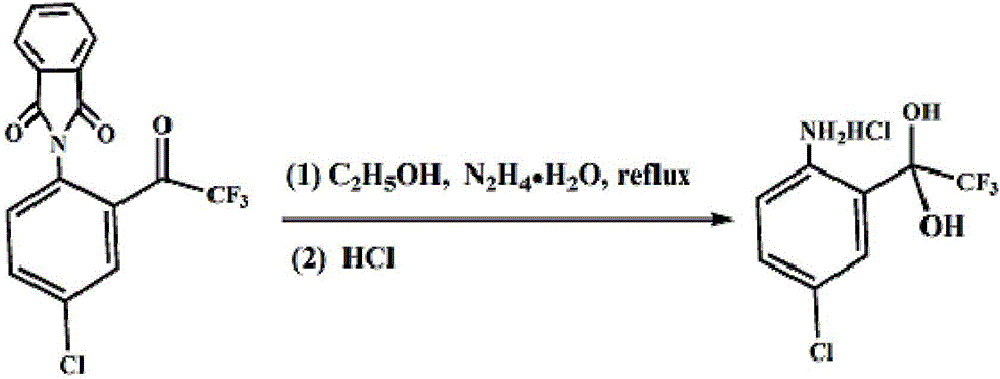Method for preparing 4-chlorine-2-(trifluoroacetyl) aniline hydrochloride hydrate
A technology of trifluoroacetylphenyl and trifluoroacetyl, which is applied in the field of compound preparation and can solve the problem of high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A method for preparing 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate, specifically comprising the steps:
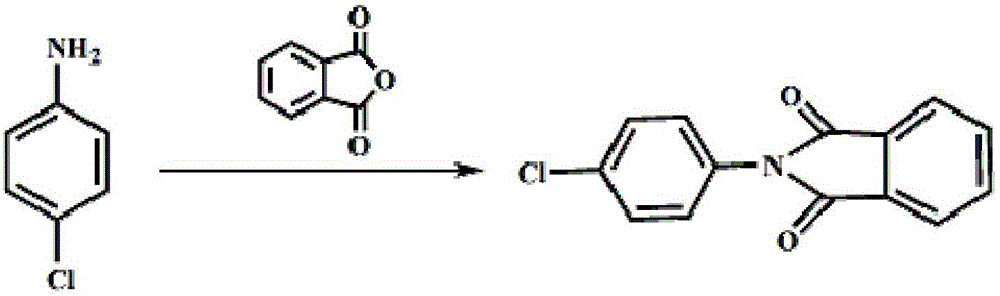
[0020] ①Synthesis of 4-chloro-N-phthalic anilide: Add 12.8g of p-chloroaniline, 102mL of toluene and 16.3g of phthalic anhydride to the reactor connected to the water separator, heat up to 110-120°C, Reflux for 1.5 hours, TLC monitoring, the reaction is complete, lower the temperature to 30-35°C, add 50mL of water, separate layers, wash the toluene layer with 100mL of water twice, cool down to -5-0°C, stir for 2 hours, suction filter, filter The cake was rinsed with an appropriate amount of water, drained, and the solid was vacuum-dried at 70°C to obtain 23.2 g of 4-chloro-N-phthalanilide, with a yield of 95.1% and a content of 99.2%.
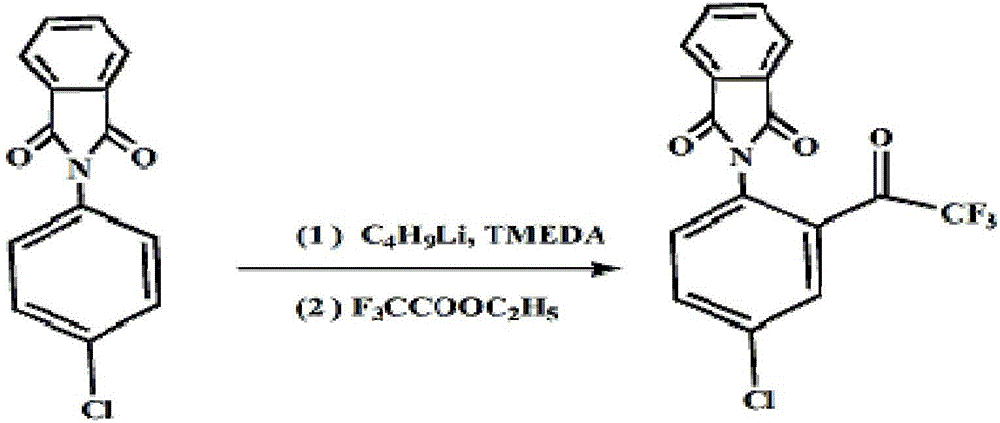
[0021] ② Synthesis of N-(4-chloro-2-trifluoroacetylphenyl)-phthalamide: In the reactor, under the protection of nitrogen, add 25.8g of 4-chloro-N-phthalamide , 258mL of methyl tert-butyl ether, 11.6g of tetramethylethyl...
Embodiment 2
[0024] A method for preparing 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate, specifically comprising the steps:
[0025] ①Synthesis of 4-chloro-N-phthalic anilide: Add 12.8g of p-chloroaniline, 102mL of toluene and 14.8g of phthalic anhydride to the reactor connected to the water separator, heat up to 110-120°C, Reflux for 1.5 hours, TLC monitoring, the reaction is complete, lower the temperature to 30-35°C, add 50mL of water, separate layers, wash the toluene layer with 100mL of water twice, cool down to -5-0°C, stir for 2 hours, suction filter, filter The cake was rinsed with an appropriate amount of water, drained, and the solid was vacuum-dried at 70°C to obtain 21.9 g of 4-chloro-N-phthalanilide, with a yield of 90% and a content of 99.0%.
[0026] ② Synthesis of N-(4-chloro-2-trifluoroacetylphenyl)-phthalamide: In the reactor, under the protection of nitrogen, add 25.8g of 4-chloro-N-phthalamide , 258mL of methyl tert-butyl ether, 11.6g of tetramethylethylen...
Embodiment 3
[0029] A method for preparing 4-chloro-2-(trifluoroacetyl)aniline hydrochloride hydrate, specifically comprising the following steps, 1. the synthesis of 4-chloro-N-phthalanilide: in a water separator connected Add 12.8g of p-chloroaniline, 51mL of toluene, and 16.3g of phthalic anhydride into the reactor, raise the temperature to 110-120°C, reflux for 1.5 hours, monitor by TLC, after the reaction is complete, cool down to 30-35°C, add 50mL of water, and divide layer, the toluene layer was washed twice with 100 mL of water, cooled to -5 ~ 0 ° C, stirred for 2 hours, filtered with suction, the filter cake was rinsed with an appropriate amount of water, and dried, and the solid was vacuum-dried at 70 ° C to obtain 4- Chloro-N-phthalanilide 19.5g, yield 80%, content 98.5%.
[0030] ② Synthesis of N-(4-chloro-2-trifluoroacetylphenyl)-phthalamide: In the reactor, under the protection of nitrogen, add 25.8g of 4-chloro-N-phthalamide , 258mL of methyl tert-butyl ether, 11.6g of tetr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com