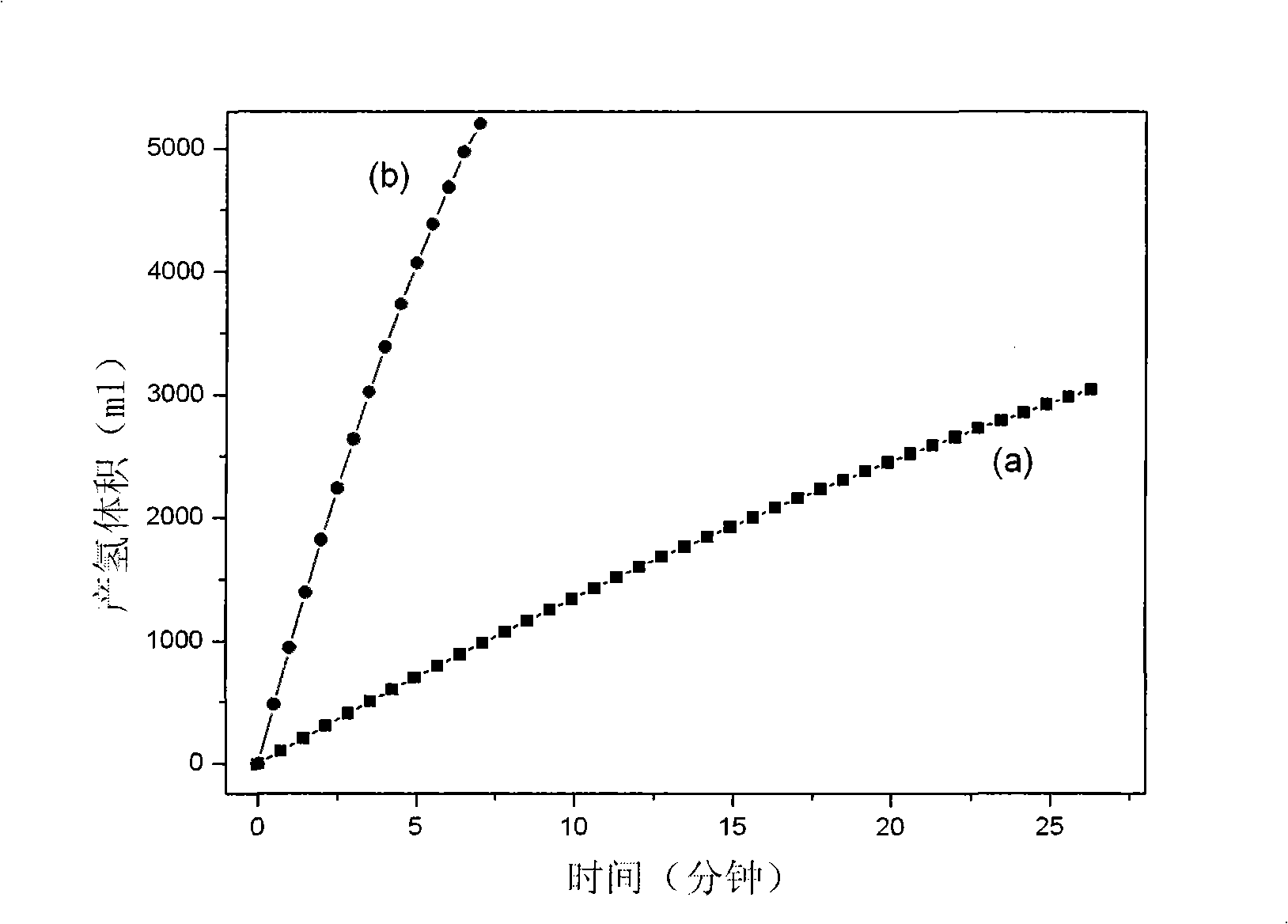Catalyst for hydrogen production by catalyzing and hydrolyzing borohydride and preparation method thereof
A borohydride and catalytic hydrolysis technology, which is applied in the fields of hydrogen production, hydrogen storage technology and materials, can solve the problems of difficult recovery of hydrolysis by-products, difficult control of catalytic hydrolysis reaction, catalyst loss, etc., so as to solve the problem of catalyst loss and realize instant control. , The effect of high preparation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation and Catalytic Performance / Structural Characterization of Nickel Foam Supported Amorphous Co-B Alloy Catalyst
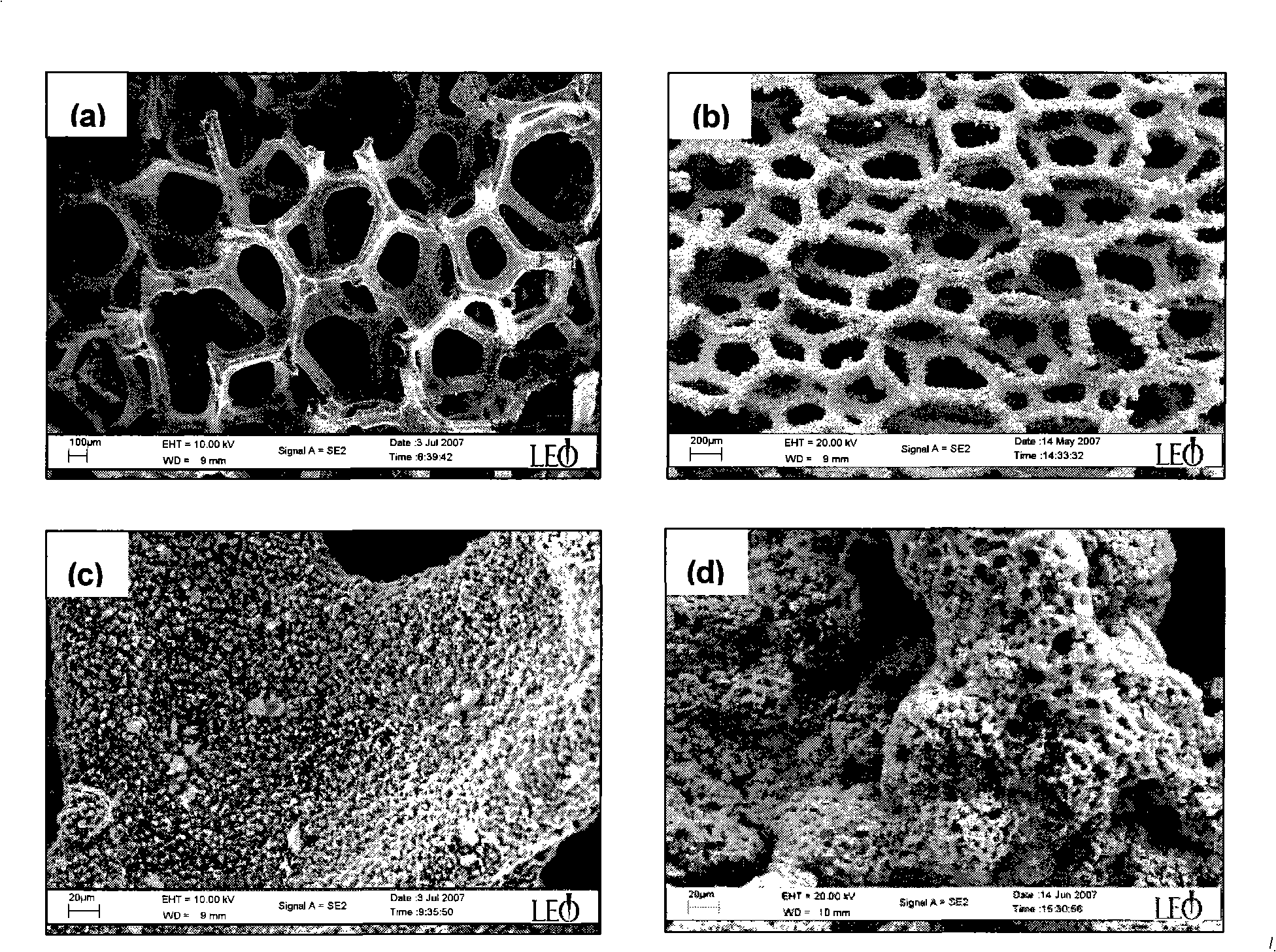
[0042] With nickel foam as the carrier, its main physical properties are: thickness 1.80mm, surface density 575g / m 2 , with a continuous through hole structure, the hole diameter is 0.20 ~ 0.50mm. Before electroless plating, nickel foam was cleaned and degreased by ultrasonic vibration in ethanol for 10 minutes, and then immersed in 10wt.% hydrochloric acid solution for activation for 1 minute. All chemical reagents used were of analytical grade without any treatment.
[0043] Improved electroless plating preparation of amorphous Co-B alloy catalyst formula:
[0044] Solution A: Cobalt Chloride (CoCl 2 ·6H 2 O) 50g / l, ammonium chloride (NH 4 Cl) 80g / l, ammonia water [NH 3 ·H 2 O (25 wt.%)] 45ml / l.
[0045] Solution B: Sodium borohydride (NaBH 4 ) 40g / l, sodium hydroxide (NaOH) 10g / l.
[0046] Operating conditions: pH value 14, re...
Embodiment 2
[0056] Embodiment 2 foamed nickel carrier electroless plating Co-Mn-B alloy is used for NaBH 4 Hydrogen production by catalytic hydrolysis
[0057] The nickel foam carrier used and the pretreatment process are the same as in Example 1.
[0058] Improve the formula of chemical plating Co-Mn-B:
[0059] Solution A: Cobalt Chloride (CoCl 2 ·6H 2 O) 60g / l, manganese chloride (MnCl 2 4H 2 O) 60g / l, ammonium chloride (NH 4 Cl) 80g / l, ethylenediamine (H 2 NCH 2 CH 2 NH 2 ) 15ml / l.
[0060] Solution B: Sodium borohydride (NaBH 4 ) 40g / l, sodium hydroxide (NaOH) 10g / l.
[0061] Preparation of Co-Mn-B / nickel foam catalyst:
[0062] The preparation process of Co-Mn-B / nickel foam catalyst is identical with embodiment 1, and prepared Co-Mn-B ternary alloy coating, through ICP chemical analysis, the content of Mn is 25wt.% in the coating, and the content of B is 9wt.%, the appearance of the coating is black, and the surface is rough and porous, with an average pore size of 2 μ...
Embodiment 3
[0065] Embodiment 3 foamed nickel carrier electroless plating Co-P alloy is used for NaBH 4 Hydrogen production by catalytic hydrolysis
[0066] The nickel foam carrier used and the pretreatment process are the same as in Example 1.
[0067] Improved formulation of electroless Co-P plating:
[0068] Solution A: Cobalt Acetate (Co(AC) 2 4H 2 O) 70g / l, sodium citrate (Na 3 C 6 h 5 o 7 2H 2 O) 60g / l, ammonium chloride (NH 4 Cl) 80g / l, ammonia water [NH 3 ·H 2 O (25 wt.%)] 45ml / l.
[0069] Solution B: sodium hypophosphite (NaH 2 PO 2 ·H 2 O) 70g / l, sodium hydroxide (NaOH) 10g / l.
[0070] Preparation of Co-P / foam nickel catalyst:
[0071] Put the pretreated foamed nickel carrier at 85°C in an equal volume of a mixture of solution A and solution B for improved electroless Co-P plating, and after 1 minute, add 1ml of 1M NaBH dropwise 4 or 1ml 0.02M PdCl 2 Solution induction, electroless Co-P plating, until no bubbles are generated, one electroless plating is complet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Areal density | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com