Optically active 2-hydroxyltetrahydrothienopyridine derivative as well as preparation method and use thereof
A technology of hydroxytetrahydrothiazide and phenopyridine, which is applied in the field of medicine, can solve the problems of loss of enzyme activity, reduction of oral bioavailability of clopidogrel, and high genetic mutation rate, and achieves the improvement of oral bioavailability and inhibition of platelet aggregation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Preparation of compound of formula V
[0059] Synthesis scheme
[0060]
[0061] Step 1: Synthesis of (R)-(2-chloro-phenyl)-hydroxy-acetic acid methyl ester (2)
[0062]
[0063] To a stirred solution of compound 1 (18.6 g, 0.1 mol) in methanol (100 mL) was added concentrated sulfuric acid (2 mL). The mixture was then heated at reflux 3, and the excess methanol was removed under vacuum. The oily residue was put into 200 mL of dichloromethane, and then washed with a 10% potassium carbonate aqueous solution (240 mL), the organic solvent was extracted, dried, and then concentrated under vacuum to obtain compound 2 (19 g, 95%) as colorless Of oil.
[0064] 1 H NMR: (Y0859-04567-023,CDCl 3 ,400MHz)δ7.52-7.50(m,1H),7.45-7.43(m,1H),7.39-7.32(m,2H),6.36-6.34(d,J=6.4Hz,1H),5.43-5.42( d,J=6.4Hz,1H),3.62(s,3H).
[0065] Step 2: Synthesis of (R)-(2-chloro-phenyl)-(4-nitro-benzenesulfonyloxy)-acetic acid methyl ester (4)
[0066]
[0067] At 0°C and N 2 Next, to a stirred solut...
Embodiment 2
[0083] Example 2: Preparation of compound of formula VI
[0084] Preparation scheme:
[0085]
[0086] Step 1: (S)-(2-Chloro-phenyl)-[2-(di-tert-butoxy-phosphoryloxymethoxy)-6,7-dihydro-4H-thiophene [3, Synthesis of 2-c]pyridin-5-yl]-methyl acetate (10)
[0087]
[0088] At -78°C and N 2 To the stirring solution of compound 6 (480mg, 1.41mmol) and NaI (430mg, 2.82mmol) prepared in step 3 of Example 1 in anhydrous THF (3mL) was added dropwise LHMDS (4.23mL, 1.0M in THF) , 2.85 mmol), and then the mixture was stirred at room temperature for 30 minutes, after which compound 9 (540 mg, 2.11 mmol) was added, and the mixture was stirred for another 10 hours. Use NH 4 The reaction was quenched with Cl solution and extracted with EA. Use NaHCO 3 The organic layer was washed with solution and brine, and then subjected to anhydrous Na 2 SO 4 Dry and concentrate to dryness. The residue was purified with a silica gel column (PE:EA=50:1-3:1) to obtain compound 10 (52 mg, 7%) as a white solid...
Embodiment 3
[0096] Example 3: In vivo pharmacokinetic test
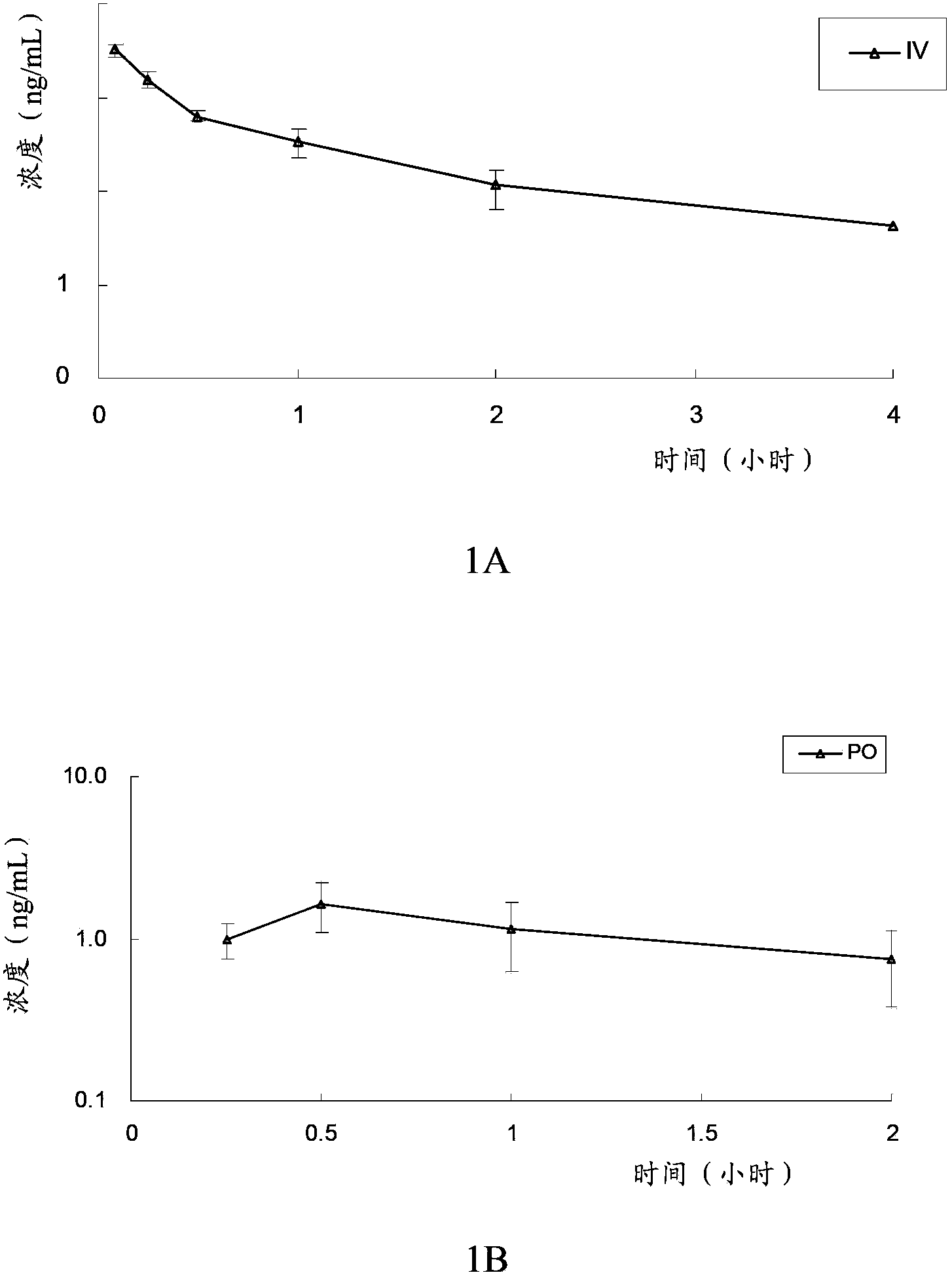
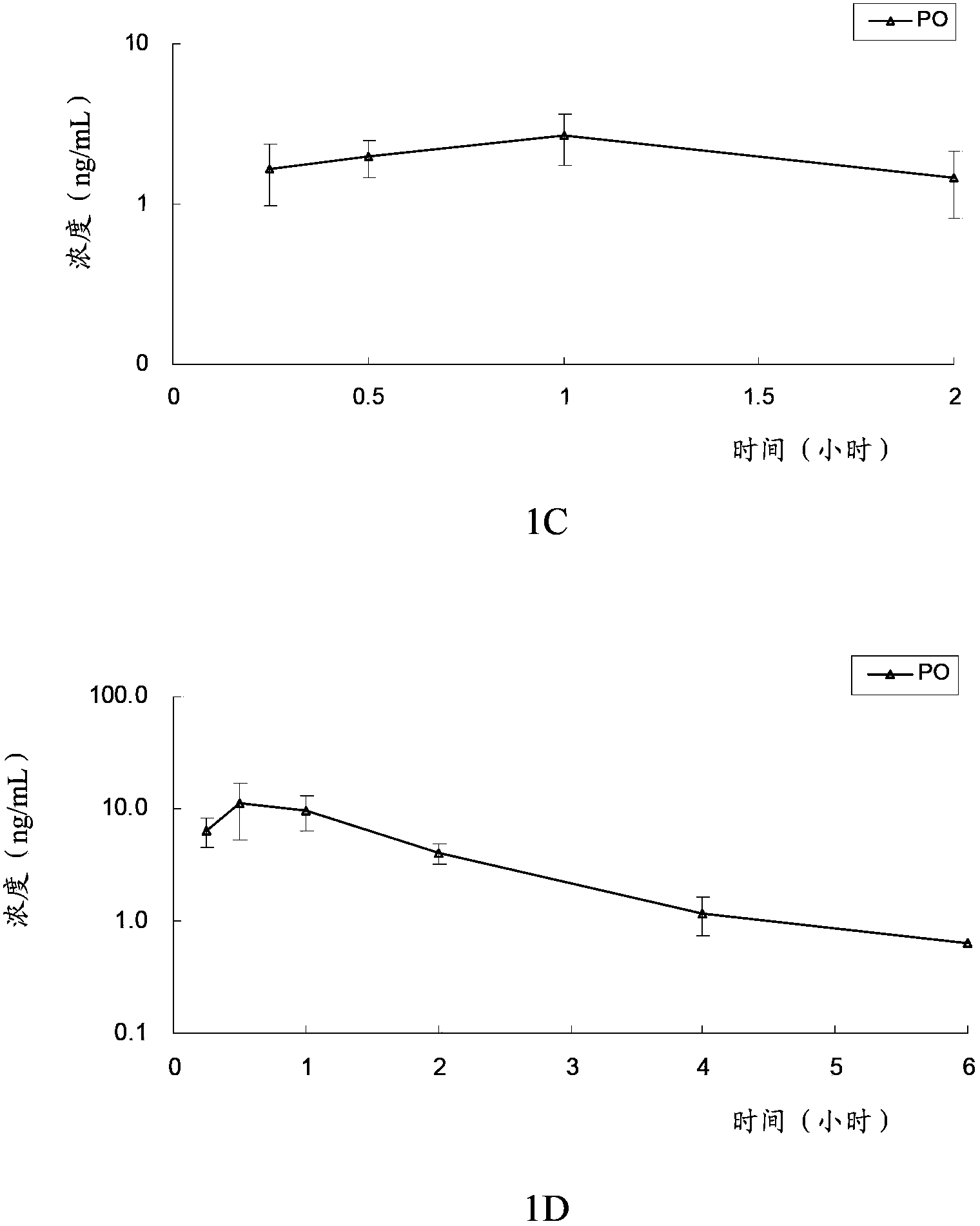
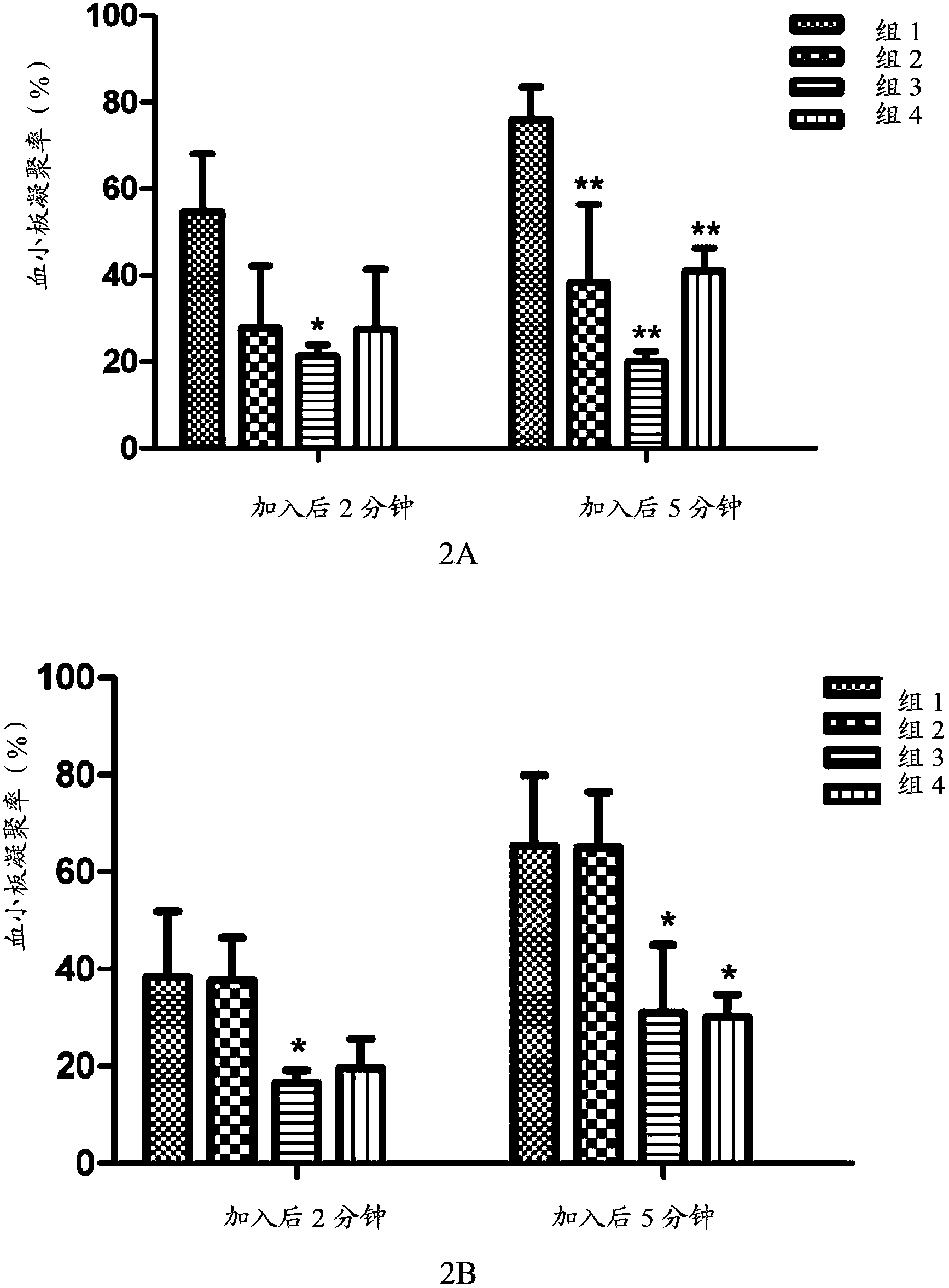
[0097] The in vivo pharmacokinetics of the compound of formula V, compound of formula VI, clopidogrel (formula VII) and the first metabolite of clopidogrel (formula VIII) of the present invention were tested. Specifically, the compound of formula V, the compound of formula VI, and clopidogrel and its first metabolite were administered orally or intravenously to rats to evaluate the pharmacokinetic characteristics of the compound of the present invention and clopidogrel in rats, and investigate The conversion of the compound of the present invention into the first metabolite in vivo, and the bioavailability of the compound of the present invention and clopidogrel was compared by measuring the plasma concentration of the first metabolite (formula VIII) in the rat at a certain time degree.
[0098] The experimental animals were male SD rats, 6 to 8 weeks old, weighing 190-215 grams, and were purchased from Beijing Weilitonghua Experim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com