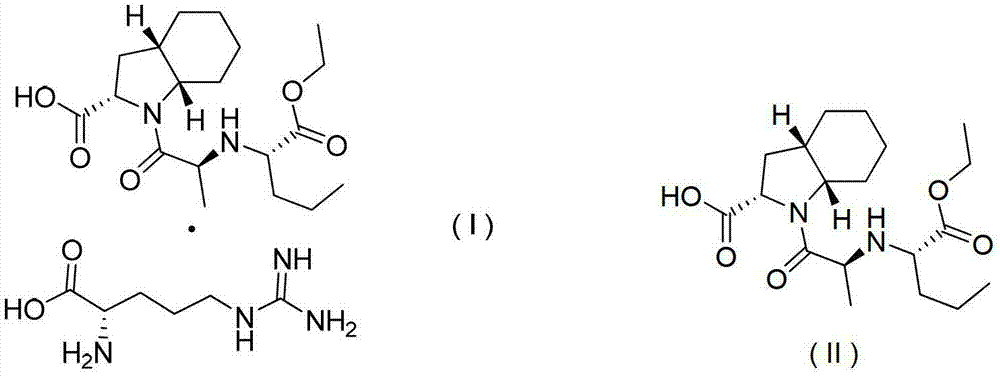Preparation method of perindopril arginine salt of gamma-crystal form
A technology of perindopril arginine salt and perindopril, which is applied to the preparation methods of peptides, chemical instruments and methods, organic chemistry and other directions, and can solve the problems of prolonging the baking time, unfavorable suction filtration, reducing yield, etc. problem, to achieve the effect of easy suction filtration and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the preparation of perindopril
[0024] Dissolve 60.0 g of perindopril tert-butylamine salt in 240 mL of water, add 240 mL of methylene chloride, stir at 25-30 ° C, then add 2.0 mol / L of hydrochloric acid dropwise to the above solution, adjust the pH value of the water phase 3.8-4.4, finally separated, extracted with dichloromethane, and the organic phase was concentrated under reduced pressure to obtain perindopril.
Embodiment 2
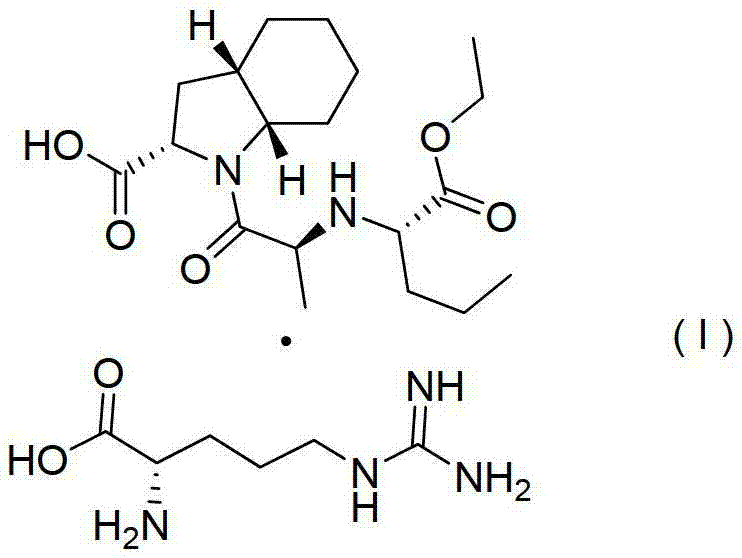
[0025] Example 2. Preparation of perindopril arginine salt (I) of γ crystal form
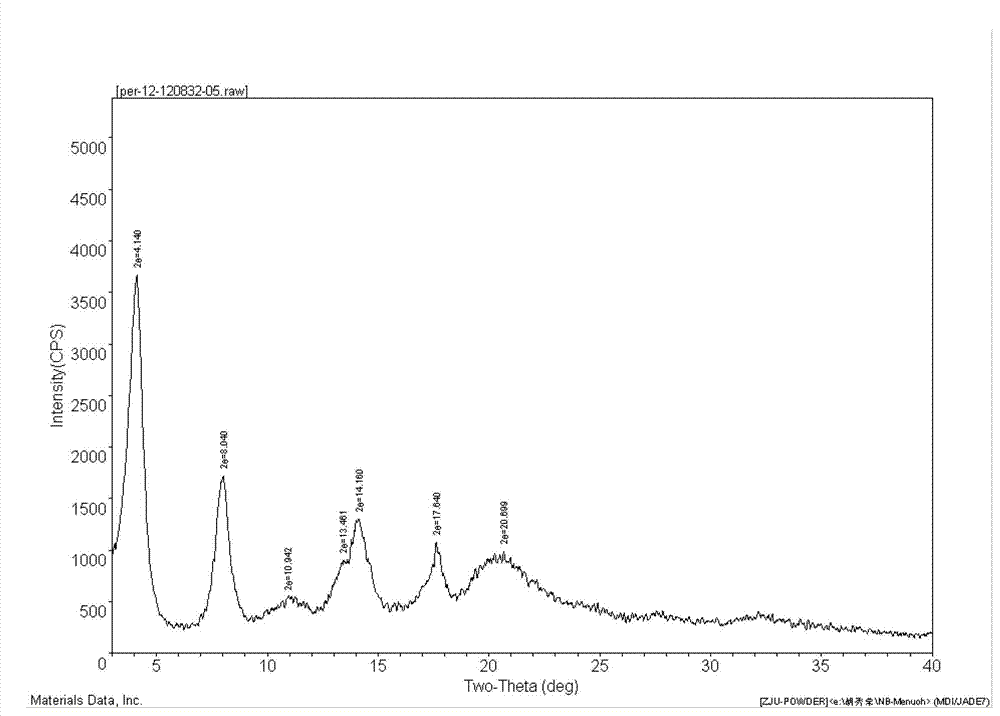
[0026] Into a 150mL four-neck flask, add 5.0g (0.0136mol) perindopril, 2.13g (0.0122mol) L-arginine, and 10mL water, and stir until dissolved. Then, 100 mL of cyclohexane was added to the above solution, and the temperature was raised and refluxed to separate water. There was no more water entering the water separator. When the solvent was no longer layered, the water separation was completed, and the temperature was lowered. After cooling to room temperature, suction filtration was started, and the white powder solid (weight 6.36 g, yield 95.9%, HPLC content: 99.98%) obtained after the filter cake was dried after suction filtration was the perindopril of the final product γ crystal form Arginine salt, X-ray powder diffraction pattern is attached figure 1 , it can be seen that the product is perindopril arginine salt of γ crystal form. The cyclohexane in the filtrate obtained by suction filtra...
Embodiment 3
[0027] Example 3. Preparation of perindopril arginine salt (I) of γ crystal form
[0028] Add 5.0 g (0.0136 mol) of perindopril, 1.66 g (0.0095 mol) of L-arginine, and 5 mL of water to a 150 mL four-neck flask, and stir until dissolved. Then, 50 mL of cyclohexane was added to the above solution, and the temperature was raised and refluxed to separate water. There is no more water entering the water separator, and the water separation is completed when the solvent is no longer stratified, and the temperature begins to cool. Cool down to 30°C and start suction filtration. After suction filtration and drying of the filter cake, the obtained white powder solid (weight 4.80 g, yield 92.8%, HPLC content: 99.95%) is the final product of perindopril in γ crystal form Arginine salt, X-ray powder diffraction pattern with attached figure 1 Consistent. The cyclohexane in the filtrate obtained by suction filtration can be recovered by simple distillation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com