Indapamide slow release tablet and its preparing method
A technology for indapamide and sustained-release tablets, applied in the field of indapamide sustained-release tablets and their preparation, can solve problems such as electrolyte and metabolic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
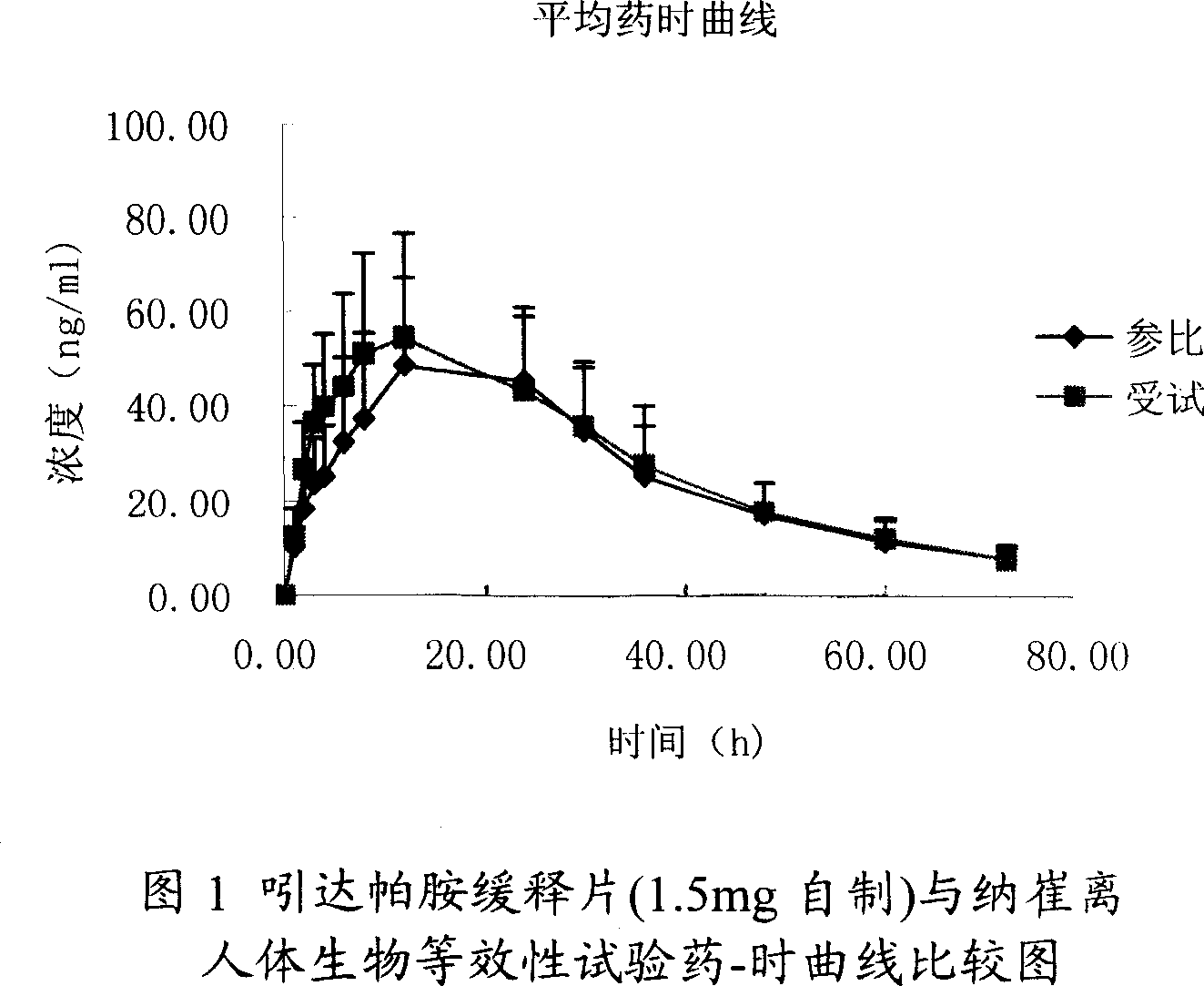
Image
Examples
Embodiment 1
[0022] Indapamide 1.50g
[0023] Hydroxypropylmethylcellulose (HPMC K4M) 37.50g
[0024] Microcrystalline Cellulose 41.25g
[0025] Lactose 30.75g
[0026] Pregelatinized starch 37.50g
[0027] Magnesium Stearate 1.50g
[0028] Made into 1000 pieces
[0029] Preparation process Pass indapamide and all auxiliary materials through a 100-mesh sieve. First, indapamide and lactose are diluted and mixed uniformly at a ratio of 1:10, and then the mixture of each excipient, indapamide and lactose is passed through a 40-mesh sieve and mixed evenly according to the prescription amount, and then the powder is directly mixed. Compressed into 7mm tablets, that is, too.
Embodiment 2
[0031] Indapamide 1.5g
[0032] Hydroxypropyl Cellulose 38.0g
[0033] Microcrystalline Cellulose 63.4g
[0034] Lactose 11.2g
[0035] Copovidone (KOLLIDON VA64) 7.5g
[0036] Pregelatinized starch 38.0g
[0037] Silica 10.0g
[0038] Made into 1000 pieces
[0039] Preparation process Pass indapamide and all auxiliary materials through a 60-mesh sieve. First, indapamide and lactose are diluted and mixed uniformly at a ratio of 1:10, and then the mixture of each excipient, indapamide and lactose is passed through a 40-mesh sieve and mixed evenly according to the prescription amount, and then the powder is directly mixed. Compressed into 7mm tablets, that is, too.
Embodiment 3
[0041] Indapamide 1.50g
[0042] HPMC K15M 37.50g
[0043] Microcrystalline Cellulose 31.25g
[0044] Lactose 40.75g
[0045] Pregelatinized starch 37.50g
[0047] Made into 1000 pieces
[0048] Preparation process Pass indapamide and all auxiliary materials through a 100-mesh sieve. First, indapamide and lactose are diluted and mixed uniformly at a ratio of 1:10, and then the mixture of each excipient, indapamide and lactose is passed through a 60-mesh sieve and mixed evenly according to the prescription amount, and then the powder is directly mixed. Compressed into 7mm tablets, that is, too.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com