Preparation technology of indapamide sustained-release tablets
A technology for indapamide and a preparation process, which is applied in the field of preparation of indapamide sustained-release tablets, can solve problems such as difficulty in achieving sustained-release effects, lowering tablet quality, and drug dust generation, and achieves improved bioavailability , The effect of uniform tablet quality and uniform release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
[0046] Unless otherwise specified, the technical means used in the implementation examples are conventional means well known to those skilled in the art.
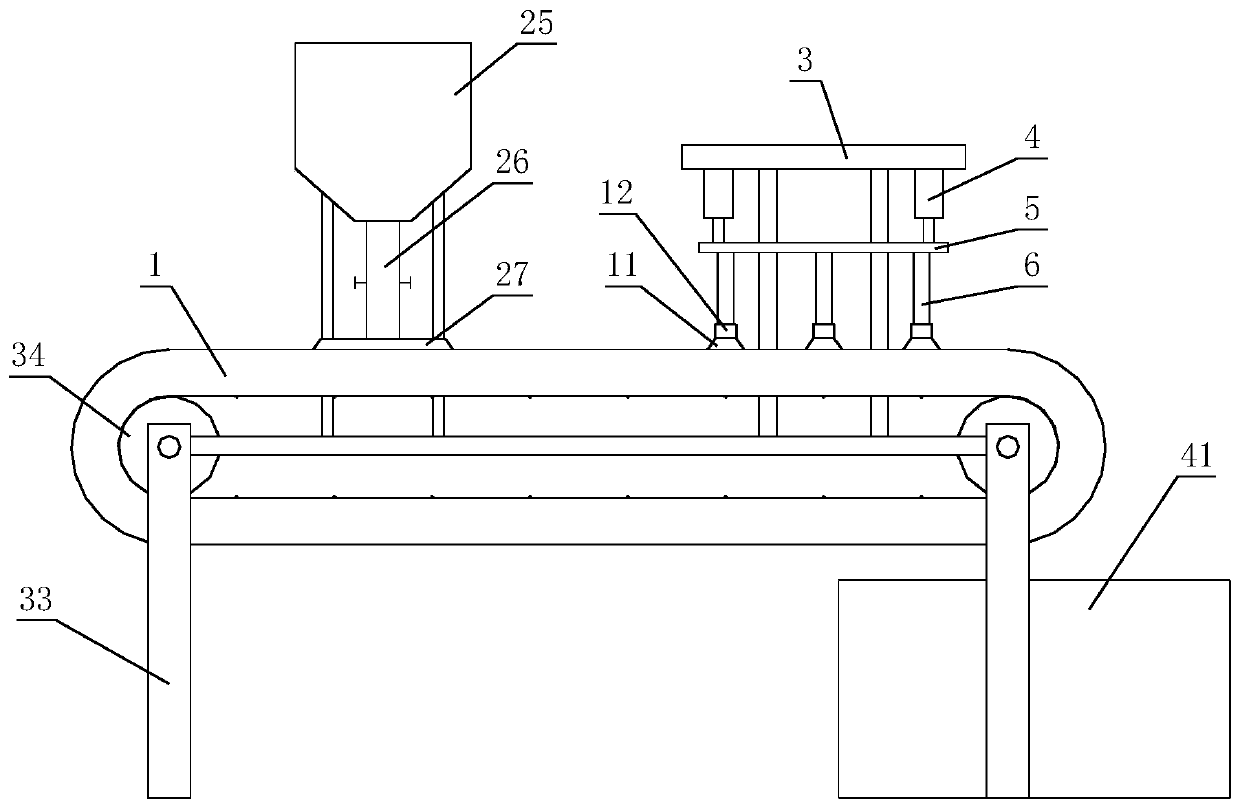
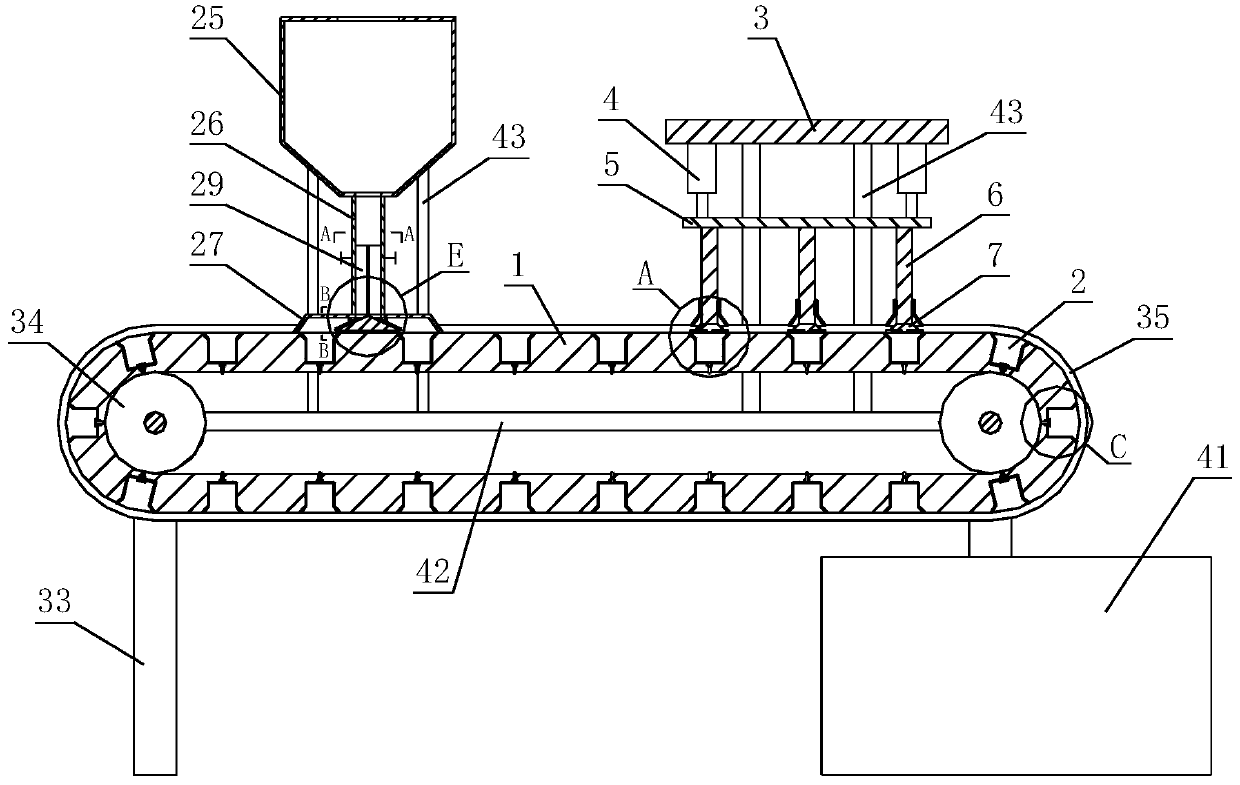
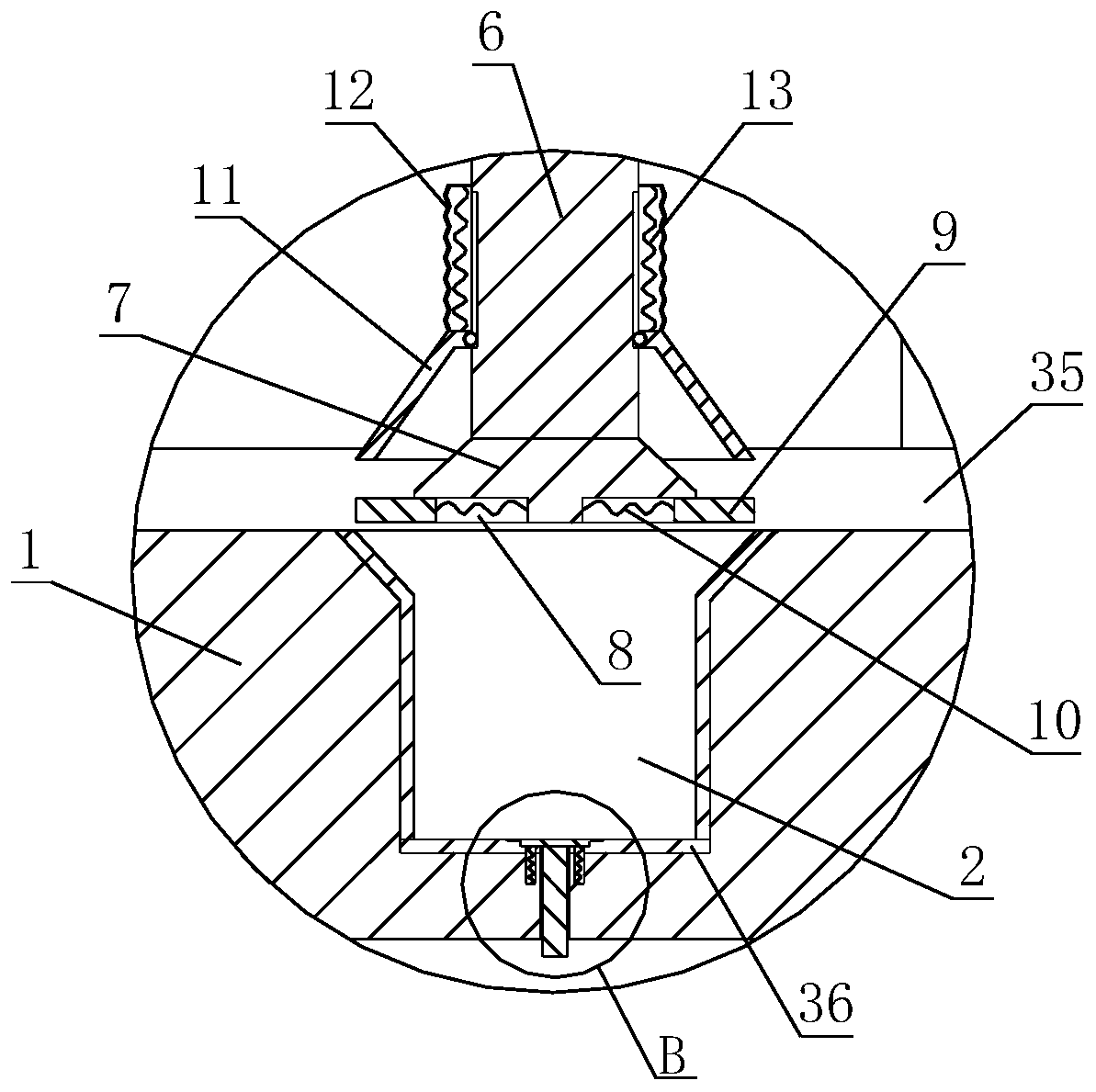
[0047] The invention discloses a preparation process of indapamide sustained-release tablets, which comprises the following steps:
[0048] (1) Slow-release materials, fillers, lubricants and indapamide are sequentially added to the powder mixer for stirring and mixing;
[0049] (2) In the process of stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com