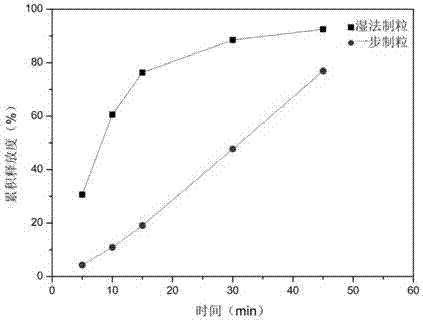
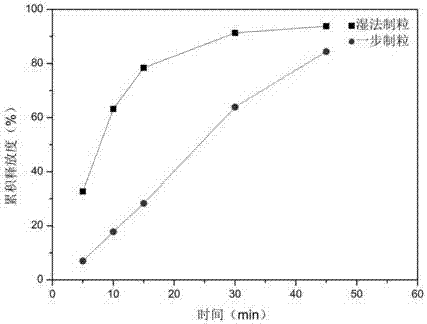
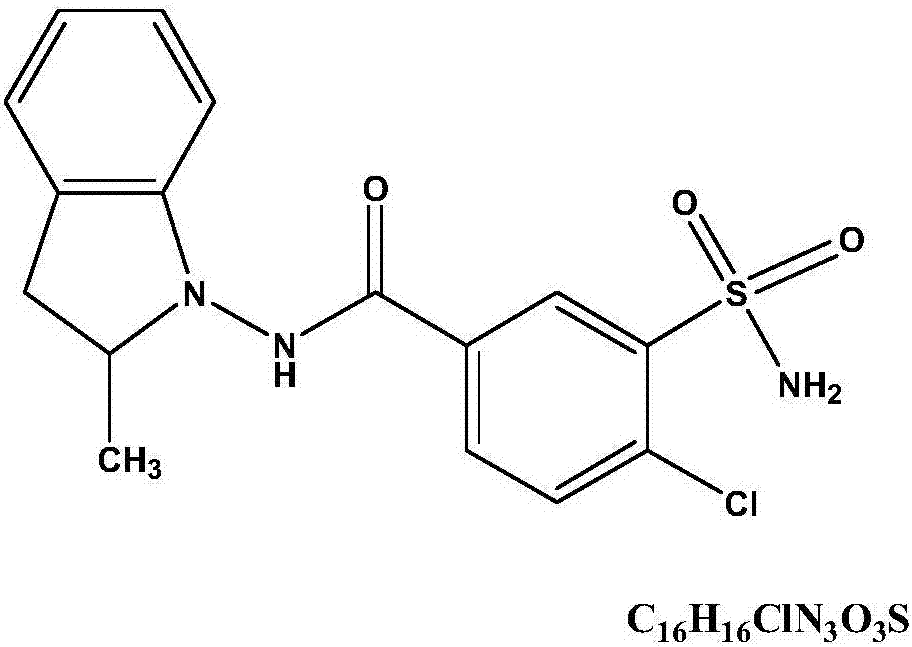
Indapamide tablet and preparation method thereof
A technology of indapamide tablets and indapamide, which is applied in the field of medicine, can solve the problems of excessive burst release, consumption, and destruction of the slow-release function of hypromellose, and achieves the effects of high cost efficiency and cost saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Material weighing: indapamide accounted for 0.05kg, lactose accounted for 1.2kg, starch accounted for 0.315kg, povidone (K30) accounted for 0.10kg, and the binder was starch slurry and PVP-K30 For the mixed solution, talcum powder accounts for 0.07kg, and magnesium stearate accounts for 0.01kg.
[0039] (2) Preparation of binder (mixed solution of starch slurry and PVP): prepare 5% starch solution, stir and boil, 10% PVP-k30, stir and swell, mix the two solutions and add micronized indapa Amine 50g, stirring spray granulation.
[0040] (3) One-step granulation process:
[0041] Weigh the talc powder and magnesium stearate and pour it into the fluidized bed, and use the side spray method for one-step granulation. The parameters of the fluidized bed during the granulation process are as follows: fluidized bed fan (HZ): 18-24; Liquid supply speed (HZ): 14~20; air inlet temperature (℃): 100~120; material temperature (℃): 30~36; equilibrium temperature (℃): 35~40; atom...
Embodiment 2
[0047] (1) Material weighing: indapamide accounted for 0.022kg, lactose accounted for 1.57kg, starch accounted for 0.27kg, povidone (K30) accounted for 0.176kg, and the binder was starch slurry and PVP-K30 For the mixed solution, talcum powder accounts for 0.124kg, and magnesium stearate accounts for 0.033kg.
[0048] (2) Preparation of binder (starch slurry and PVP mixed solution): prepare 5% starch solution, stir and boil, 10% PVP-k30, stir and swell, add micronized API 22g after the two solutions are mixed, Agitated spray granulation.
[0049] (3) One-step granulation process:
[0050] Weigh the talc powder and magnesium stearate and pour it into the fluidized bed, and use the side spray method for one-step granulation. The parameters of the fluidized bed during the granulation process are as follows: fluidized bed fan (HZ): 18-24; Liquid supply speed (HZ): 14~20; air inlet temperature (℃): 100~120; material temperature (℃): 30~36; equilibrium temperature (℃): 35~40; atom...
Embodiment 3
[0054] (1) Material weighing: indapamide accounted for 0.11kg, lactose accounted for 1.43kg, starch accounted for 0.44kg, povidone (K30) accounted for 0.11kg, and the binder was starch slurry and PVP-K30 For the mixed solution, talcum powder accounts for 0.09kg, and magnesium stearate accounts for 0.024kg.
[0055] (2) Preparation of binder (starch slurry and PVP mixed solution): prepare 5% starch solution, stir and boil, 10% PVP-k30, stir and swell, add micronized API 110g after the two solutions are mixed, Agitated spray granulation.
[0056] (3) One-step granulation process:
[0057] Weigh the talc powder and magnesium stearate and pour it into the fluidized bed, and use the side spray method for one-step granulation. The parameters of the fluidized bed during the granulation process are as follows: fluidized bed fan (HZ): 18-24; Liquid supply speed (HZ): 14~20; air inlet temperature (℃): 100~120; material temperature (℃): 30~36; equilibrium temperature (℃): 35~40; atomizat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| relative humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com