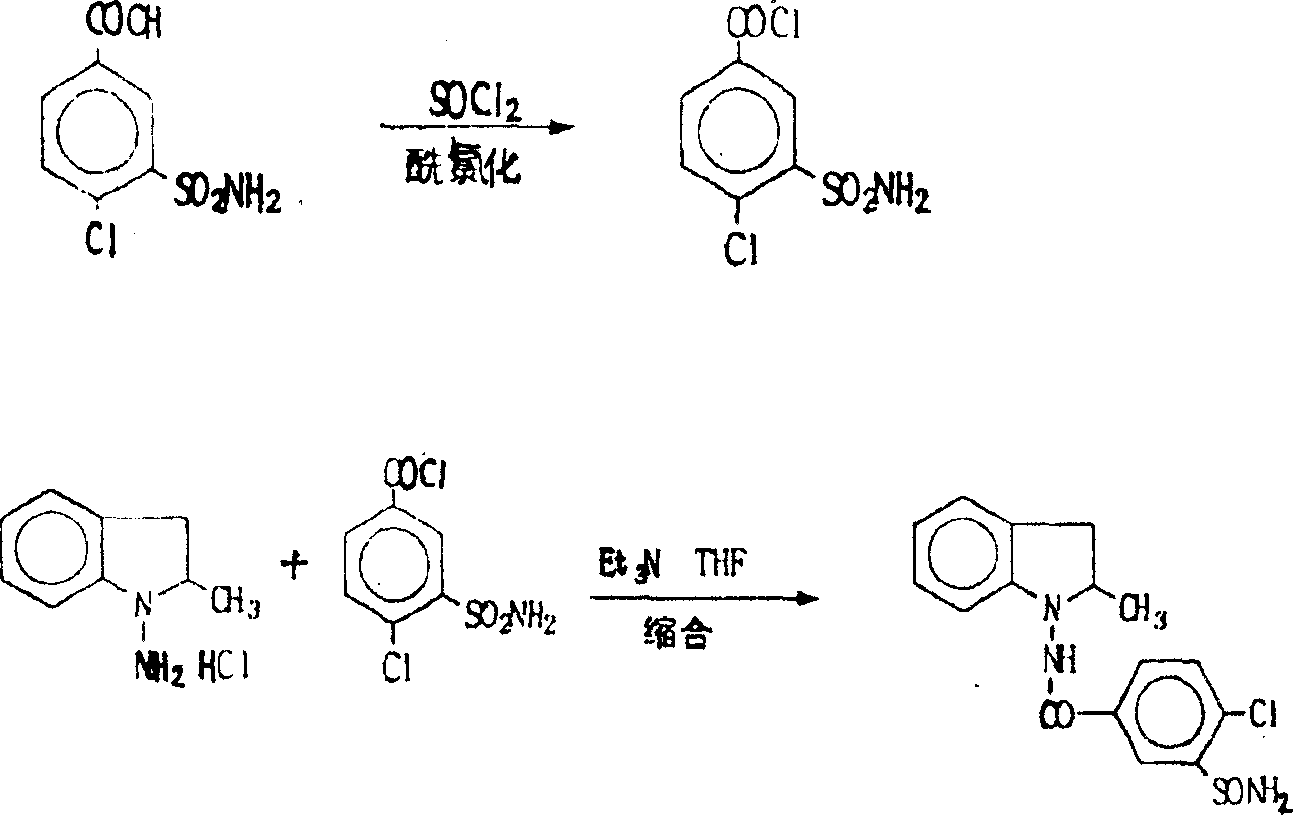
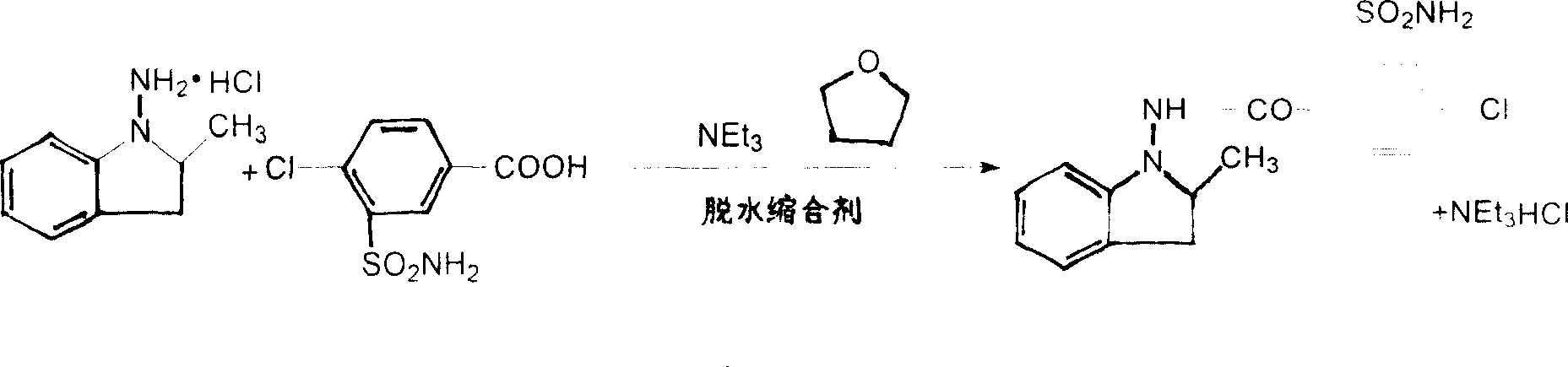
Synthesis method of indapamide
A technology of indapamide and its synthesis method, which is applied in the field of medicine and chemical industry, can solve problems such as inconvenient production operation, safety accidents, and long production cycle, and achieve the effects of reducing pollution and corrosion, improving safety, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 200ml tetrahydrofuran and 9.3g N-amino-2-methylindoline hydrochloride to the reaction flask, add 8.5ml triethylamine dropwise under stirring, cool to 10°C, add N,N'-dicyclohexyl carbon Diimine 10.8g and 4-chloro-3-sulfonylamino-benzoic acid 11.0g, the reaction temperature was controlled at 10°C, and the reaction time was 10 hours. After the reaction was completed, the insoluble matter was filtered off, the tetrahydrofuran in the filtrate was evaporated to dryness, and Isopropanol-water solution was recrystallized to obtain 16.5 g of indapamide, yield 90.4%.
Embodiment 2
[0033] Add 260ml tetrahydrofuran and 11.0g N-amino-2-methylindoline hydrochloride to the reaction flask, add 9.2ml triethylamine dropwise under stirring, cool to 10°C, add N,N'-diisopropyl 7.0 g of carbodiimide and 14.0 g of 4-chloro-3-sulfonylamino-benzoic acid, the reaction temperature was controlled at 5 ° C, and the reaction time was 20 hours. After the reaction was completed, the insoluble matter was filtered off, and the tetrahydrofuran in the filtrate was evaporated to dryness. Isopropanol-water solution was added for recrystallization to obtain 16.9 g of indapamide, yield 92.6%.
Embodiment 3
[0035] Add 100ml of dichloromethane and 9.3g of N-amino-2-methylindoline hydrochloride to the reaction flask, add 8.0ml of triethylamine dropwise under stirring, cool to 10°C, add N,N'-bicyclic Hexylcarbodiimide 10g, control the reaction temperature at 4.0°C, and the reaction time is 4 hours. After the reaction is completed, filter out the insoluble matter, evaporate the dichloromethane in the filtrate to dryness, add isopropanol-water solution for recrystallization, and obtain Inda Peramide 15.1g, yield 83.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com