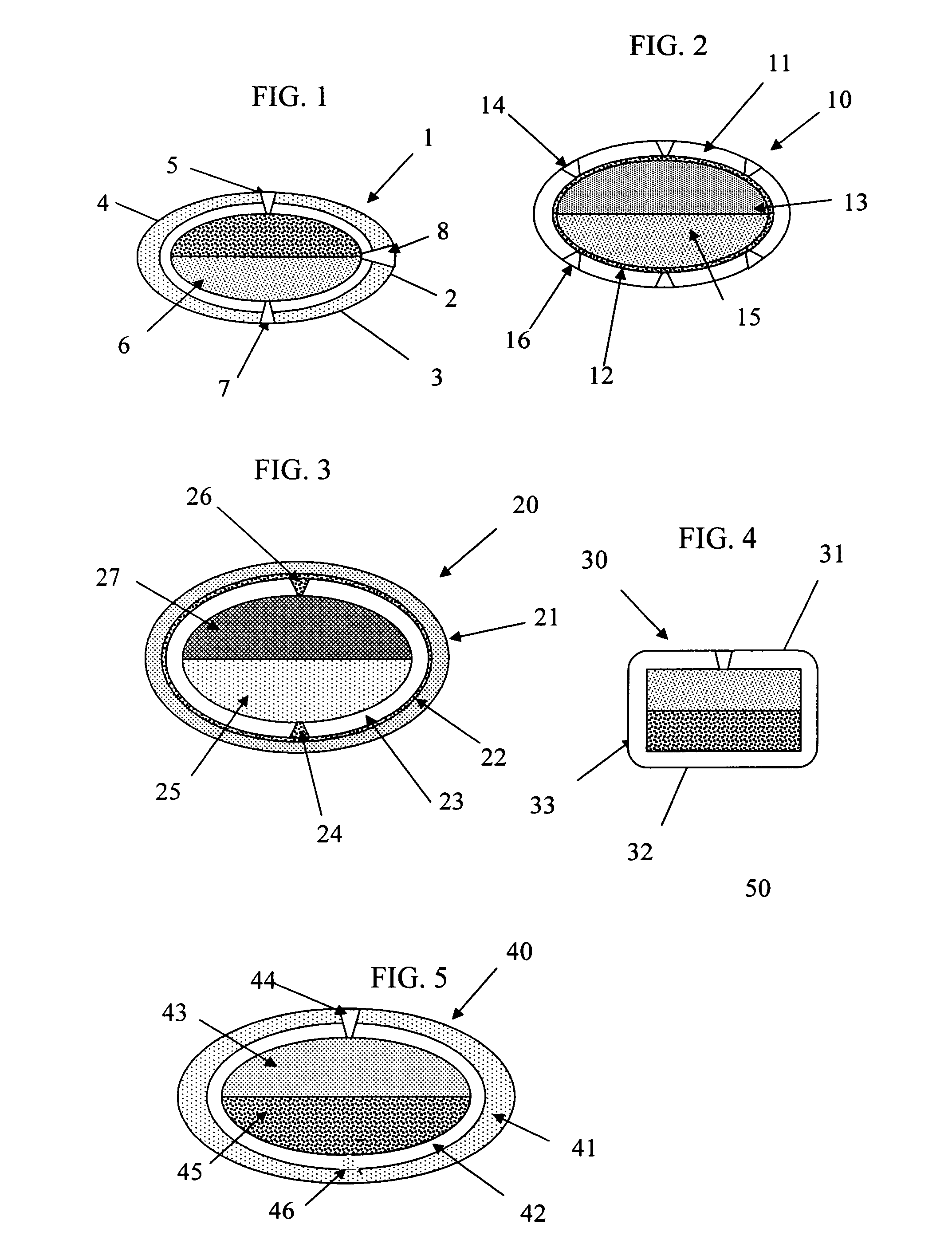
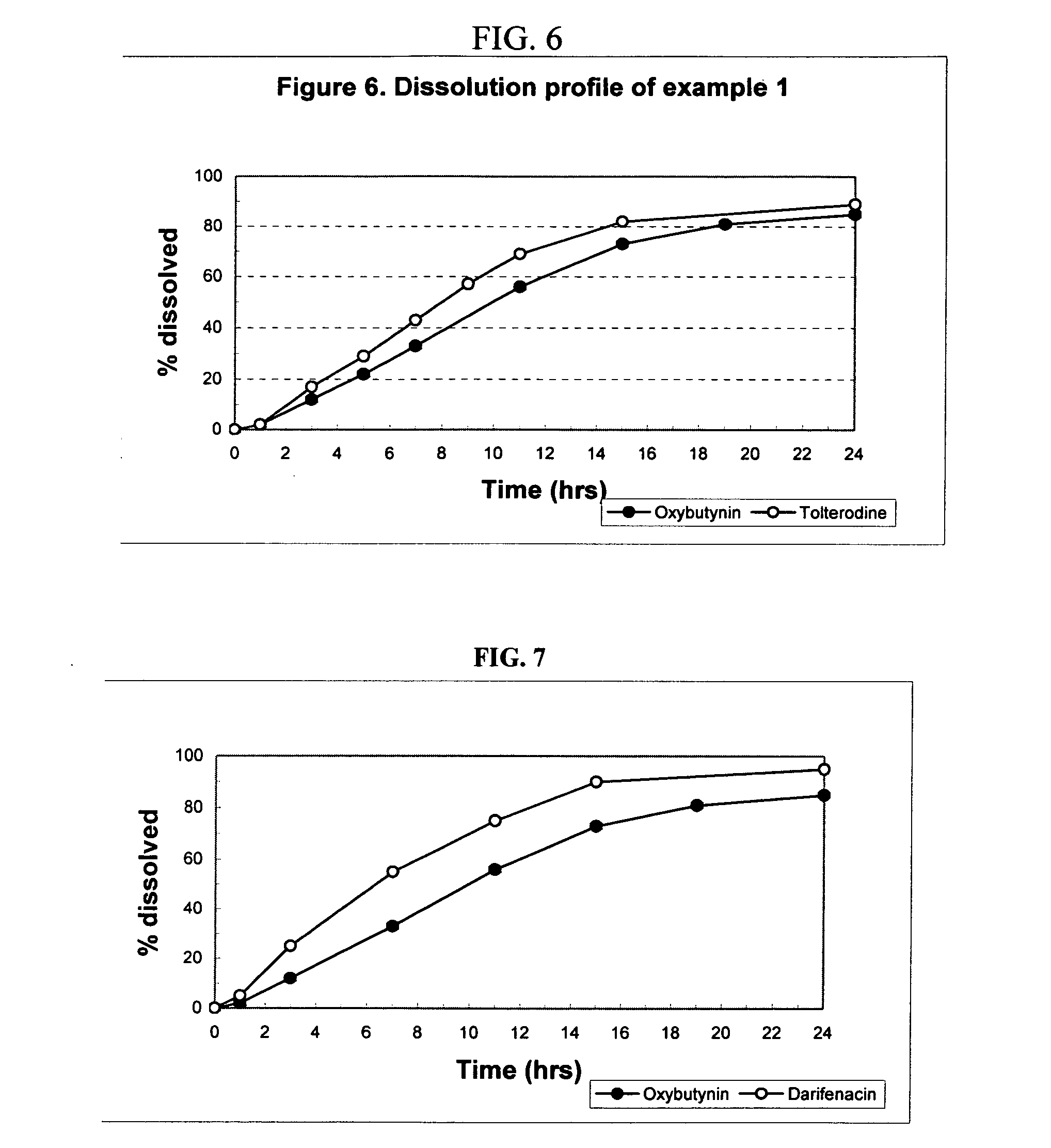
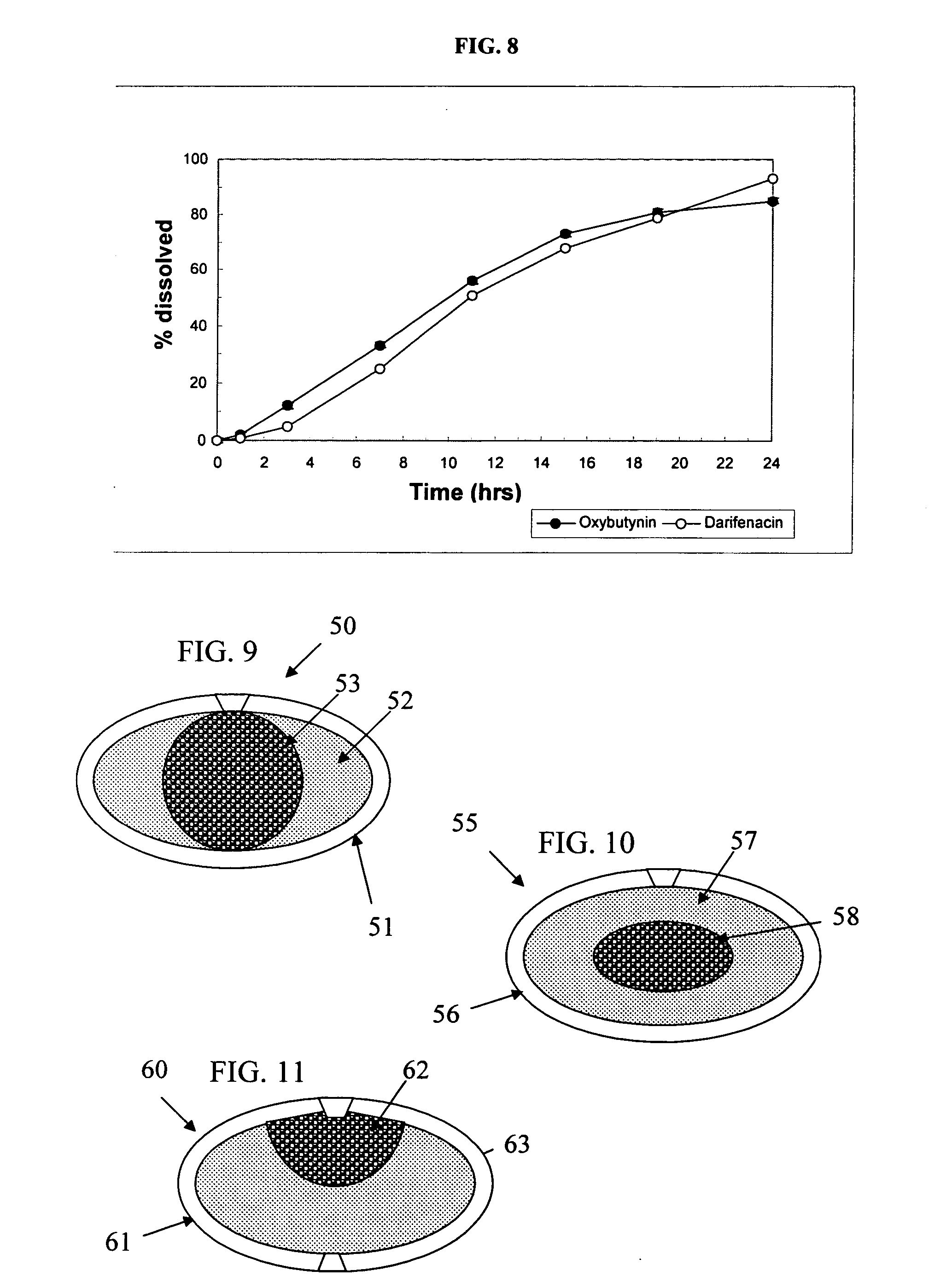
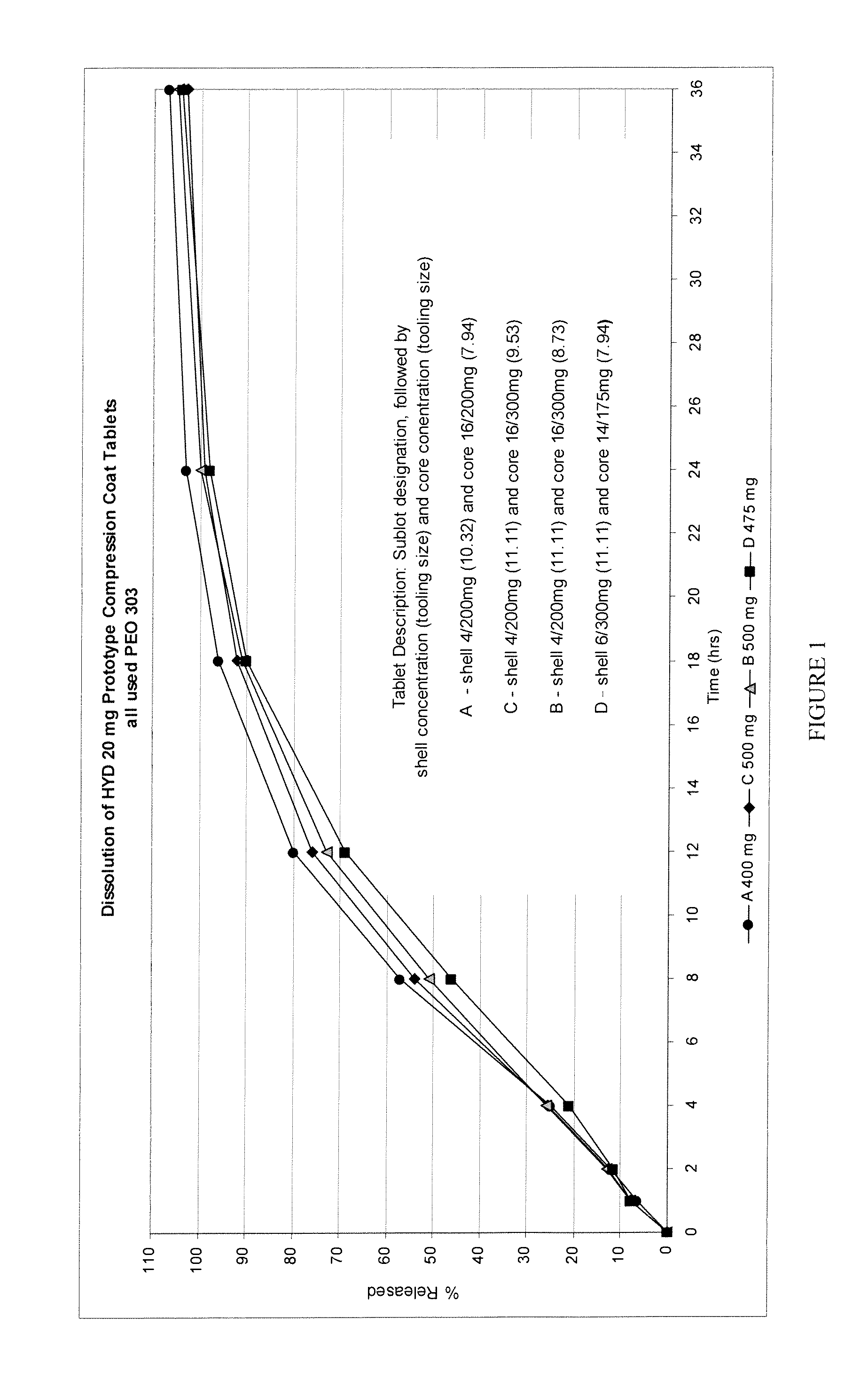
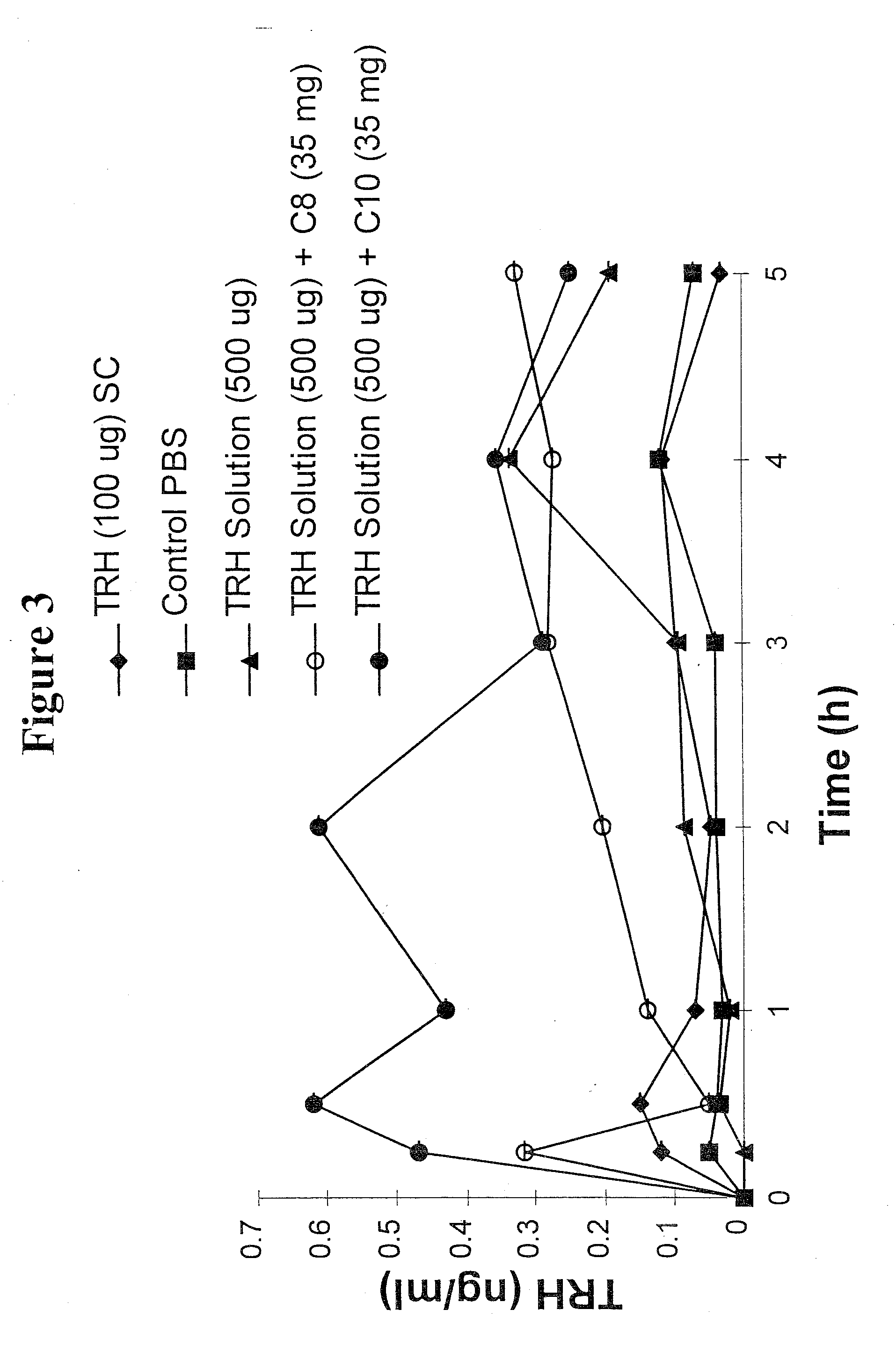
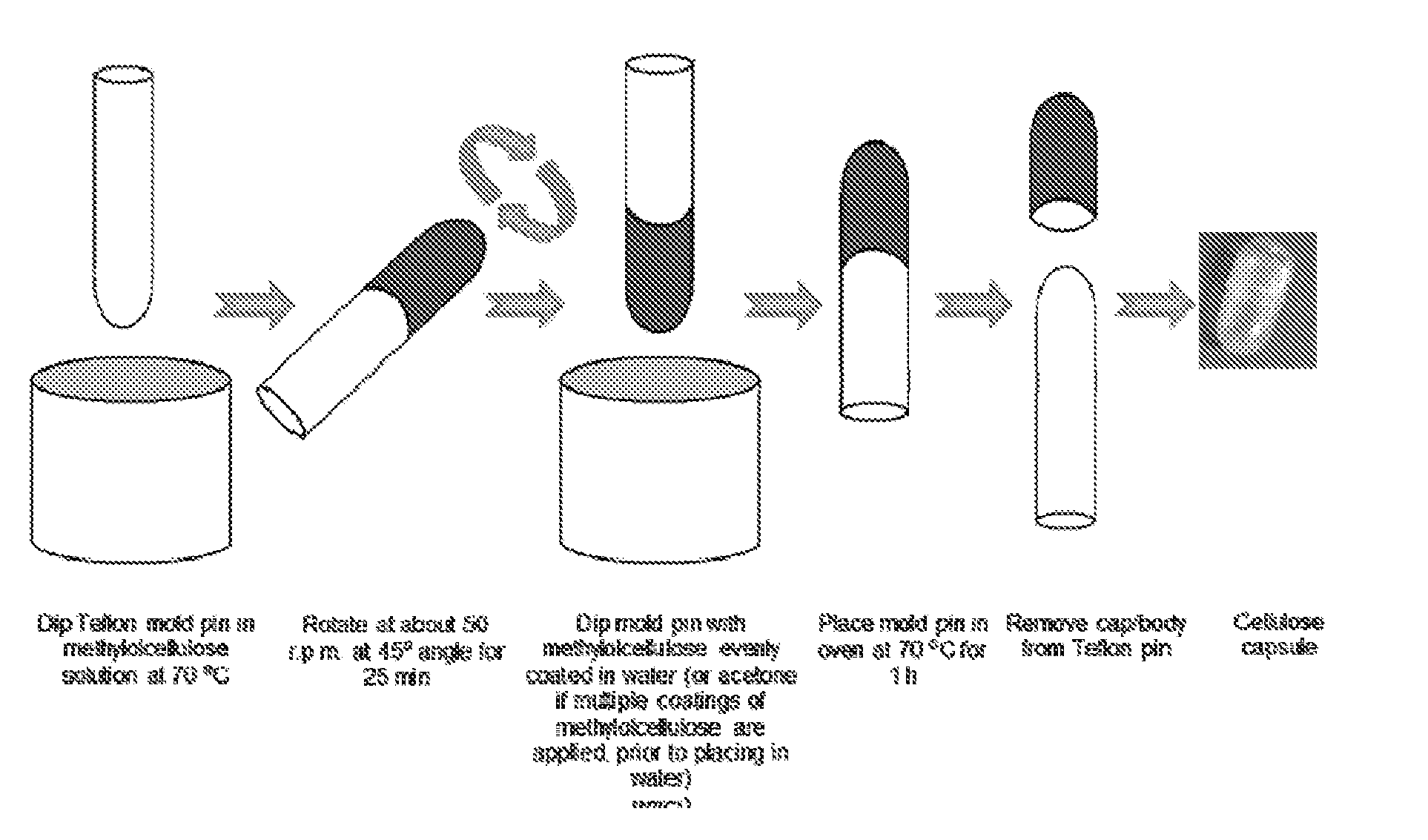
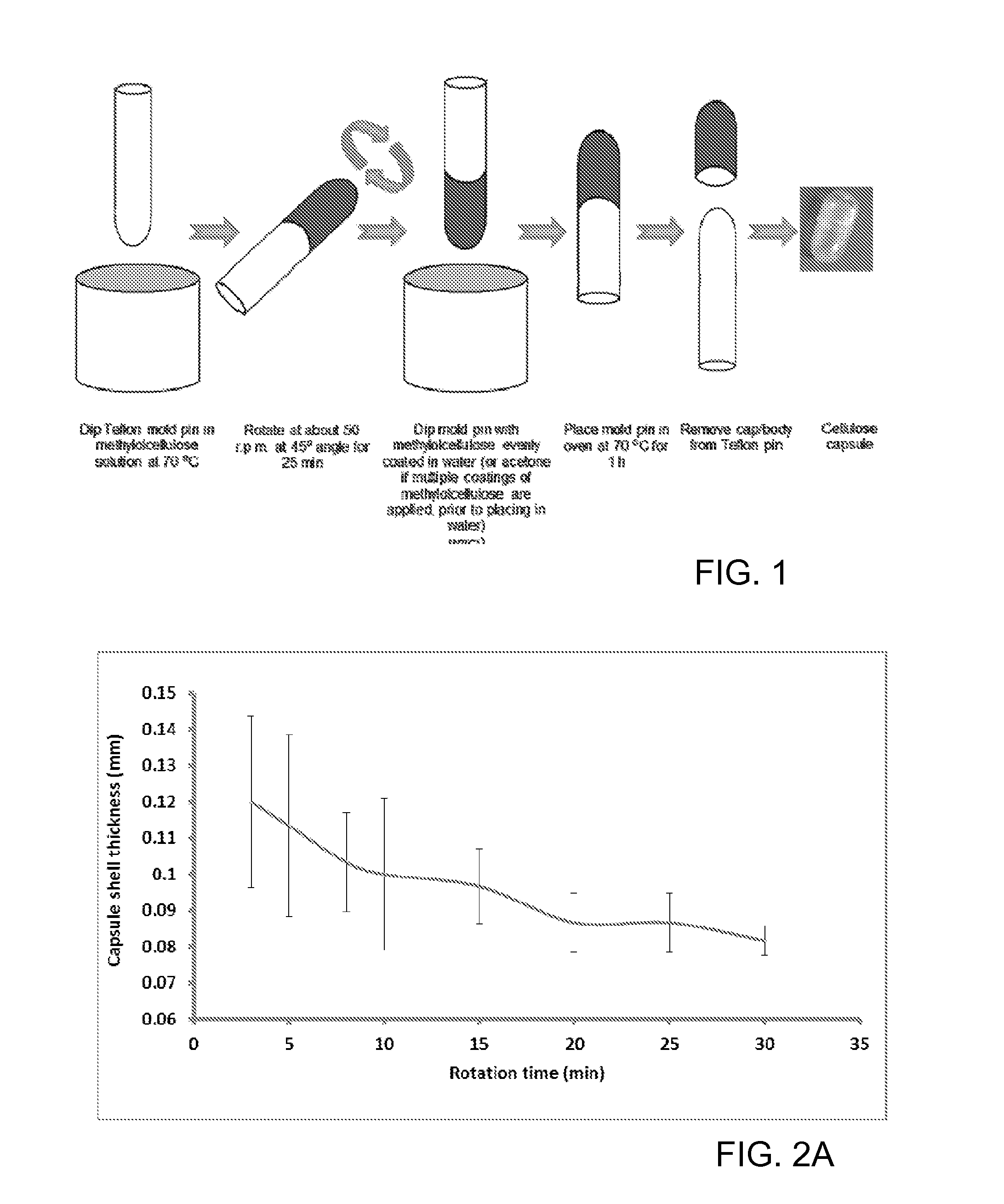
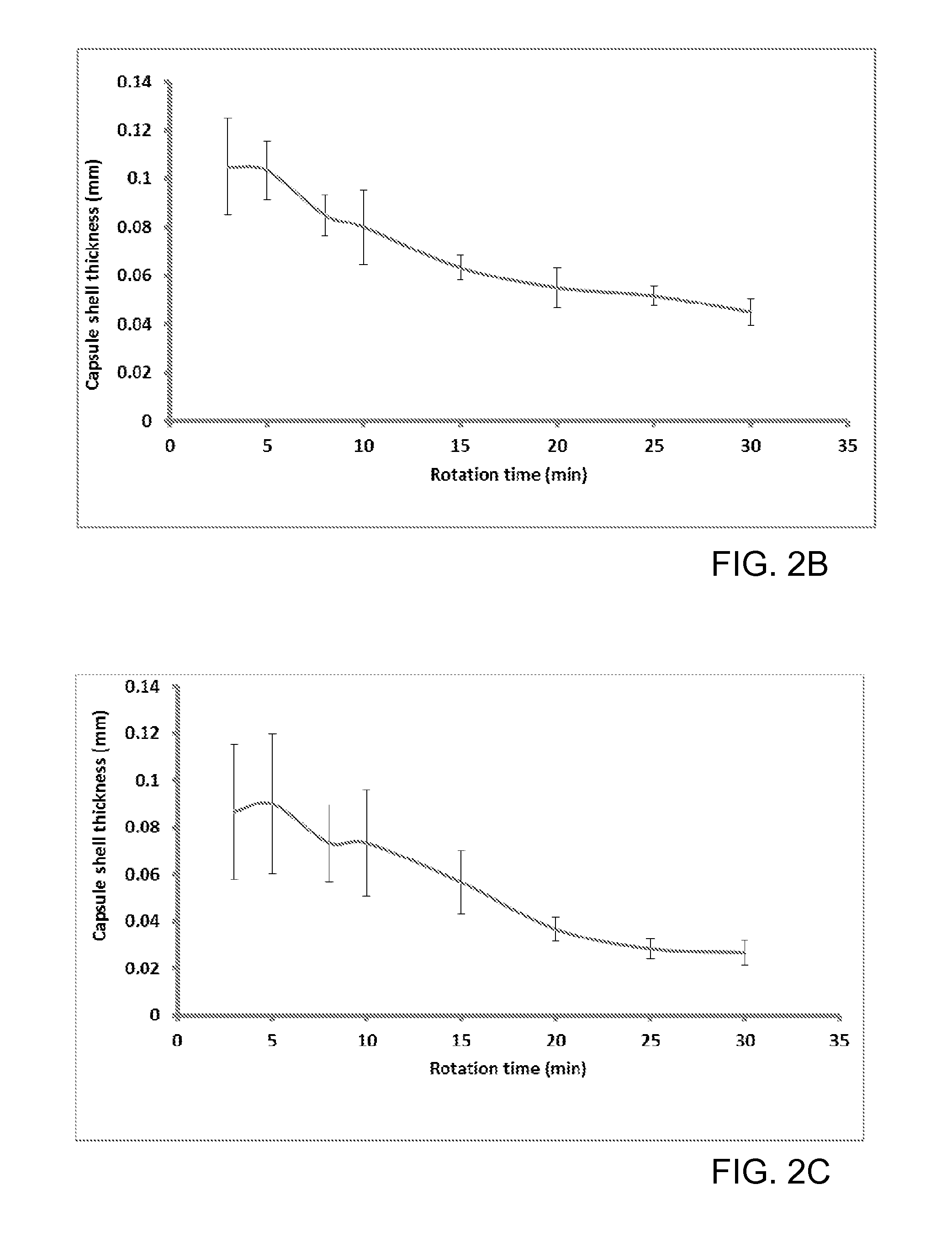
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
117 results about "Controlled Release Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
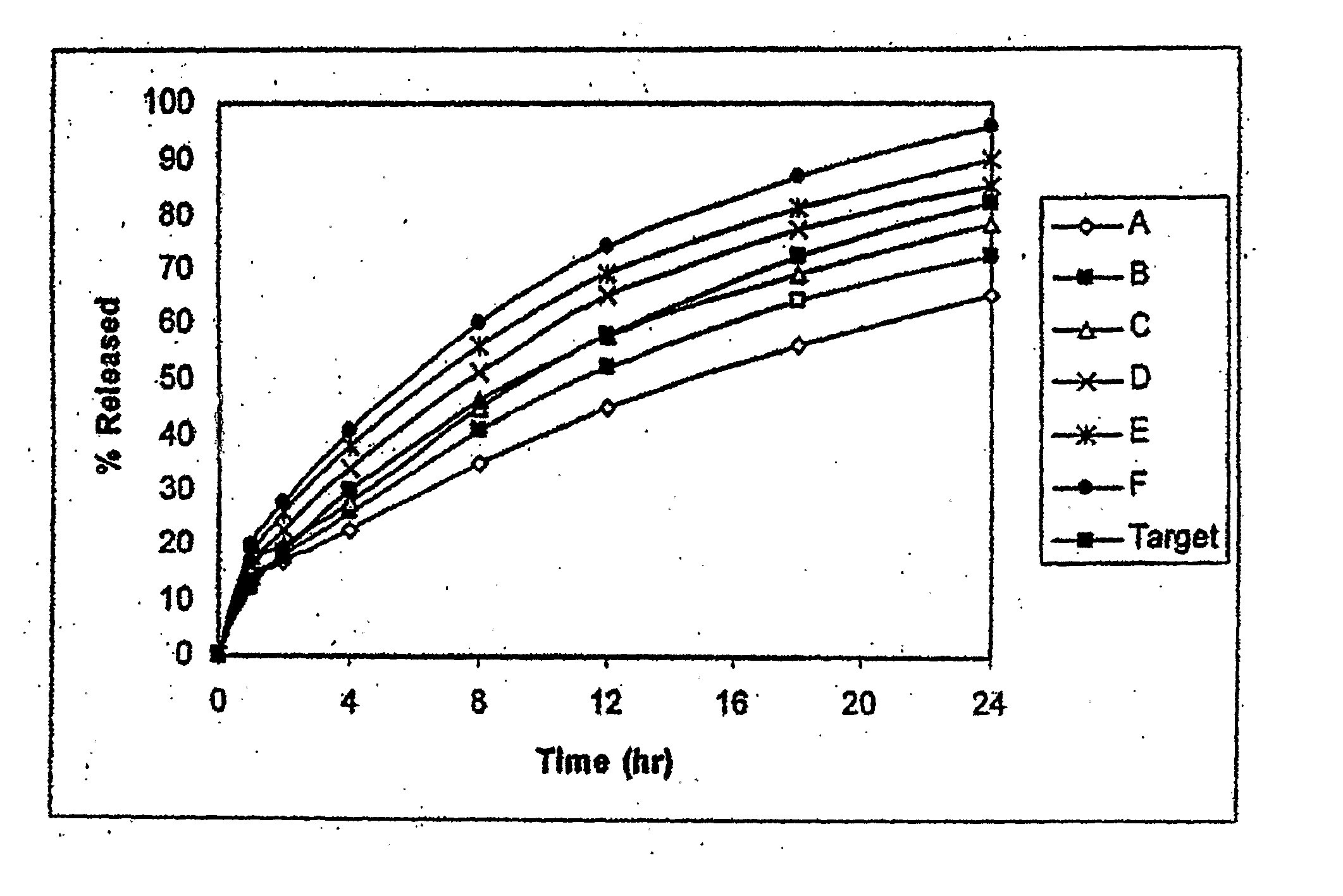
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer
InactiveUS6129933AReduce reunionLiquid surface applicatorsGranular deliveryHydrophobic polymerDissolution
A stabilized solid controlled release dosage form having a coating derived from an aqueous dispersion of ethylcellulose is obtained by overcoating a substrate including a therapeutically active with an aqueous dispersion of ethylcellulose and then curing the coated substrate at a temperature and relative humidity elevated to a suitable level above ambient conditions until the coated dosage form attains a stabilized dissolution profile substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:PURDUE PHARMA LP
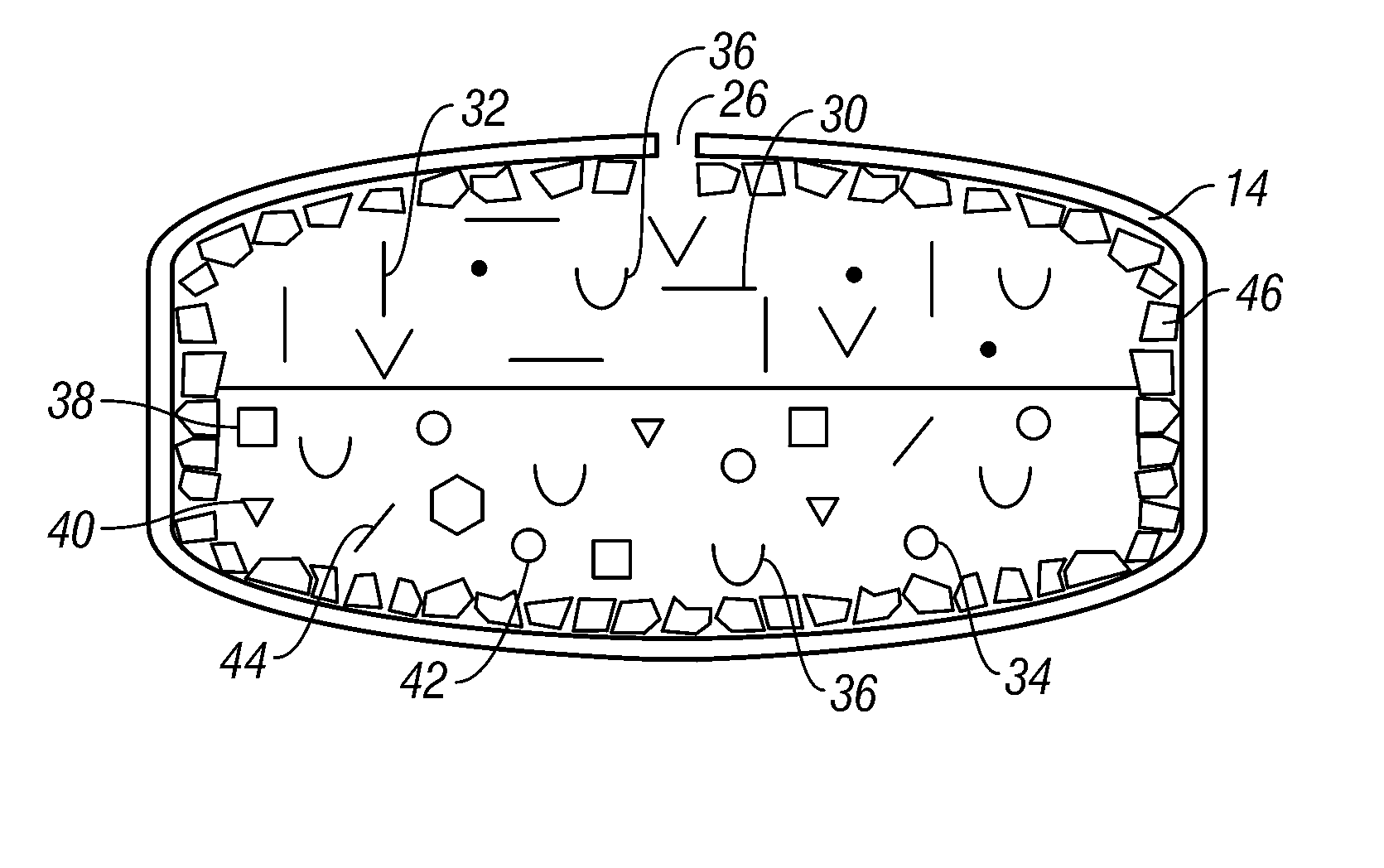
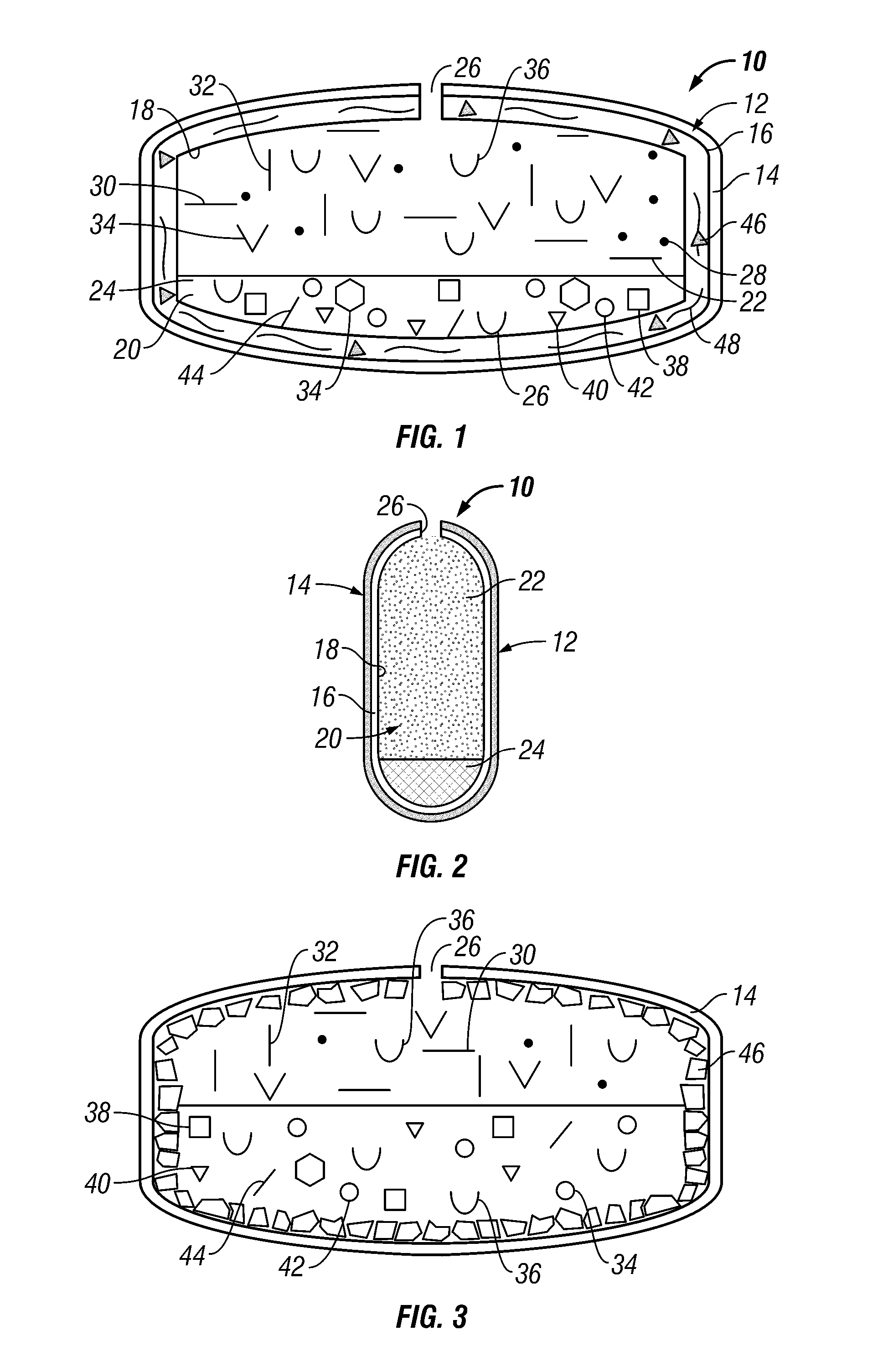
Dual controlled release dosage form
A dosage form that provides a controlled release of at least two different active agents is provided. Particular embodiments include a dosage form that provides therapeutically effective levels of a first active agent and a second active agent in a mammal for an extended period of time following oral administration. An osmotic device containing a bi-layered core is provided. The osmotic device provides a dual controlled release of both drugs from the core. The layers of the core are in stacked, substantially concentric or substantially eccentric arrangement.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Encased Tamper Resistant Controlled Release Dosage Forms
ActiveUS20120164220A1Reducing abuse potential of dosage formBiocideNervous disorderGastric fluidEnzyme
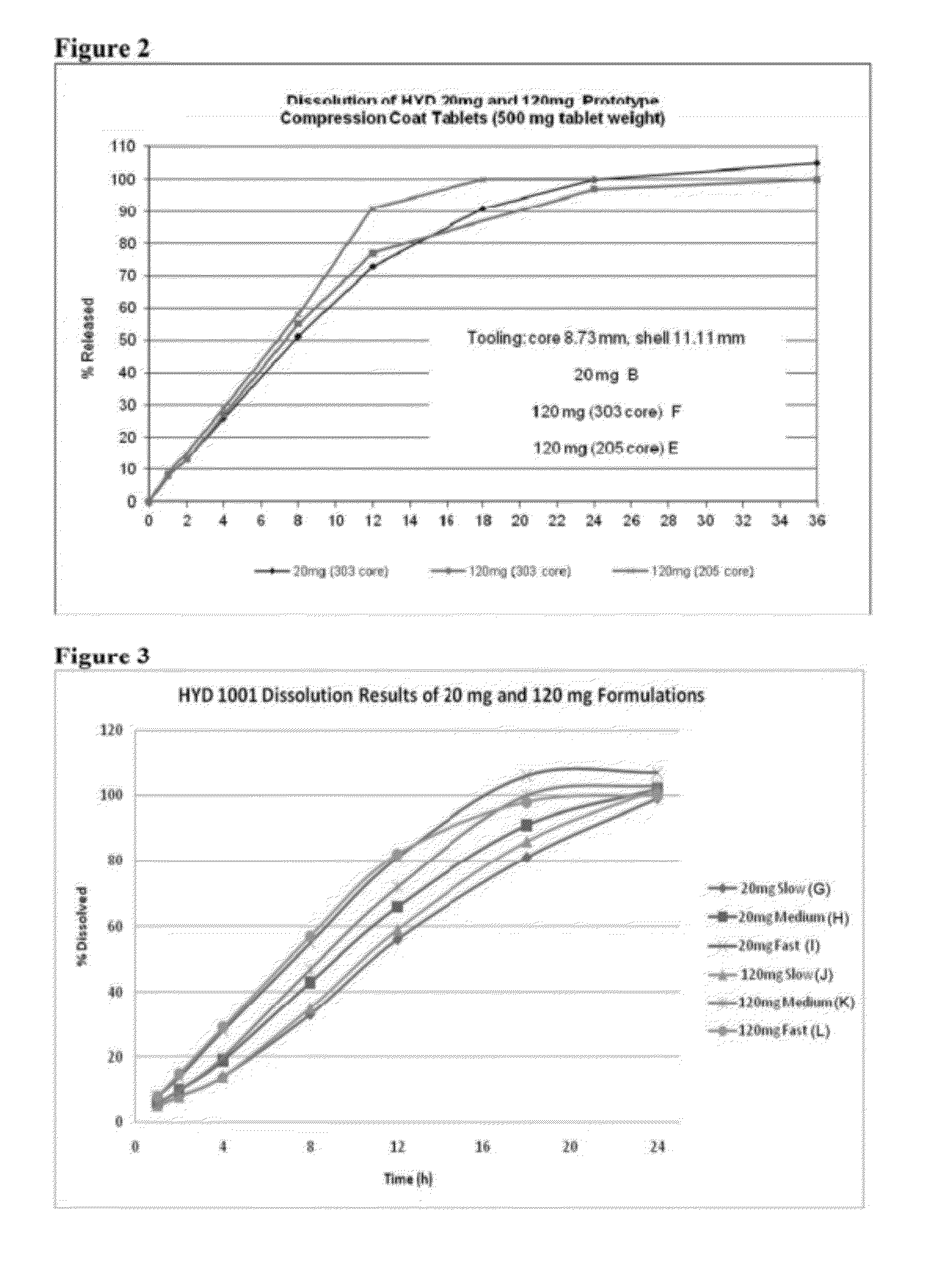
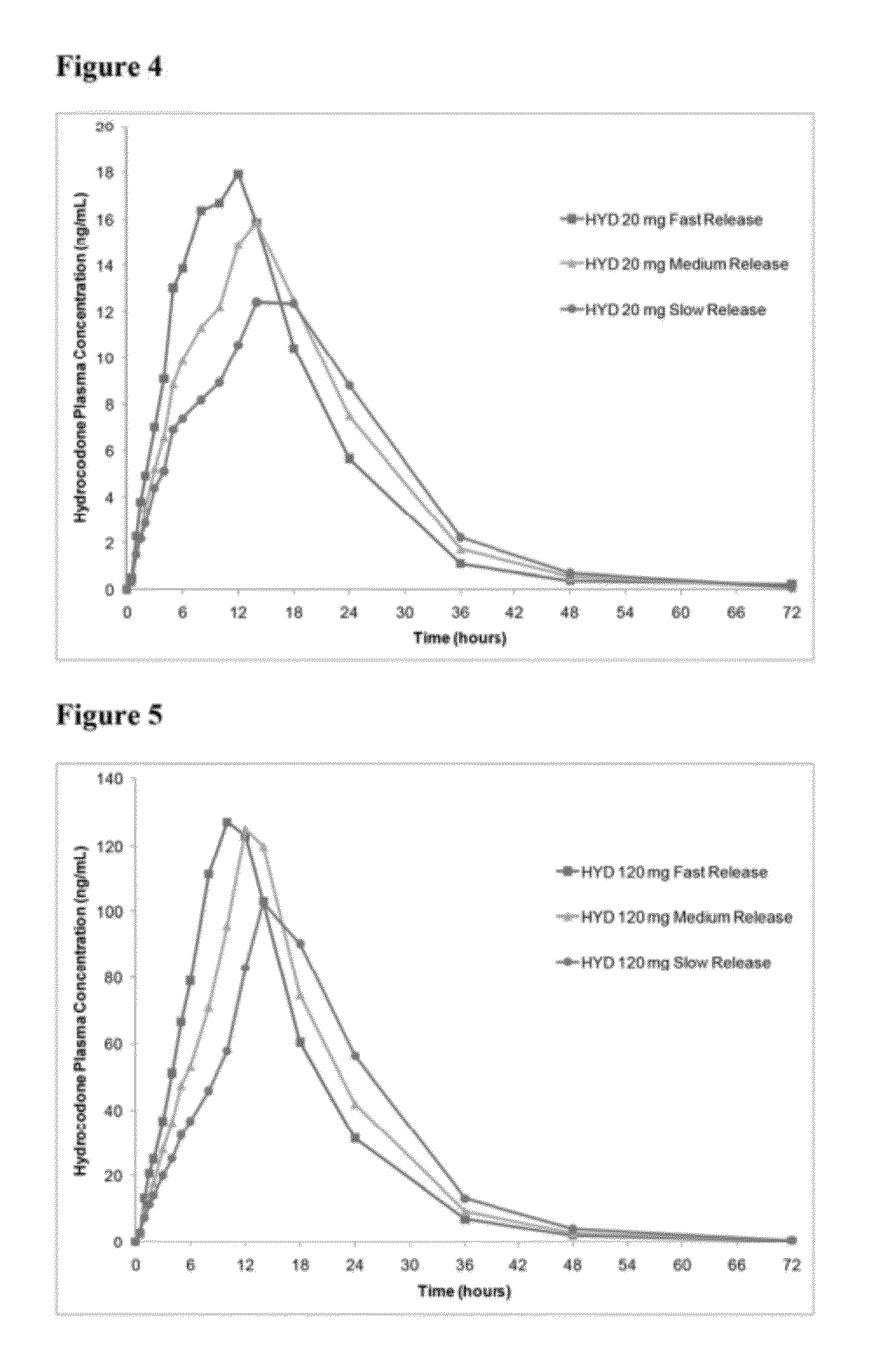
In certain embodiments, the present invention is directed to a solid controlled release dosage form comprising: a core comprising a first portion of an opioid analgesic dispersed in a first matrix material; and a shell encasing the core and comprising a second portion of the opioid analgesic dispersed in a second matrix material; wherein the amount of opioid analgesic released from the dosage form is proportional within 20% to elapsed time from 8 to 24 hours, as measured by an in-vitro dissolution in a USP Apparatus 1 (basket) at 100 rpm in 900 ml simulated gastric fluid without enzymes (SGF) at 37 C.
Owner:PURDUE PHARMA LP
Solid oral dosage form containing an enhancer
InactiveUS8119159B2Minimizes risk of local irritationImprove oral bioavailabilityBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
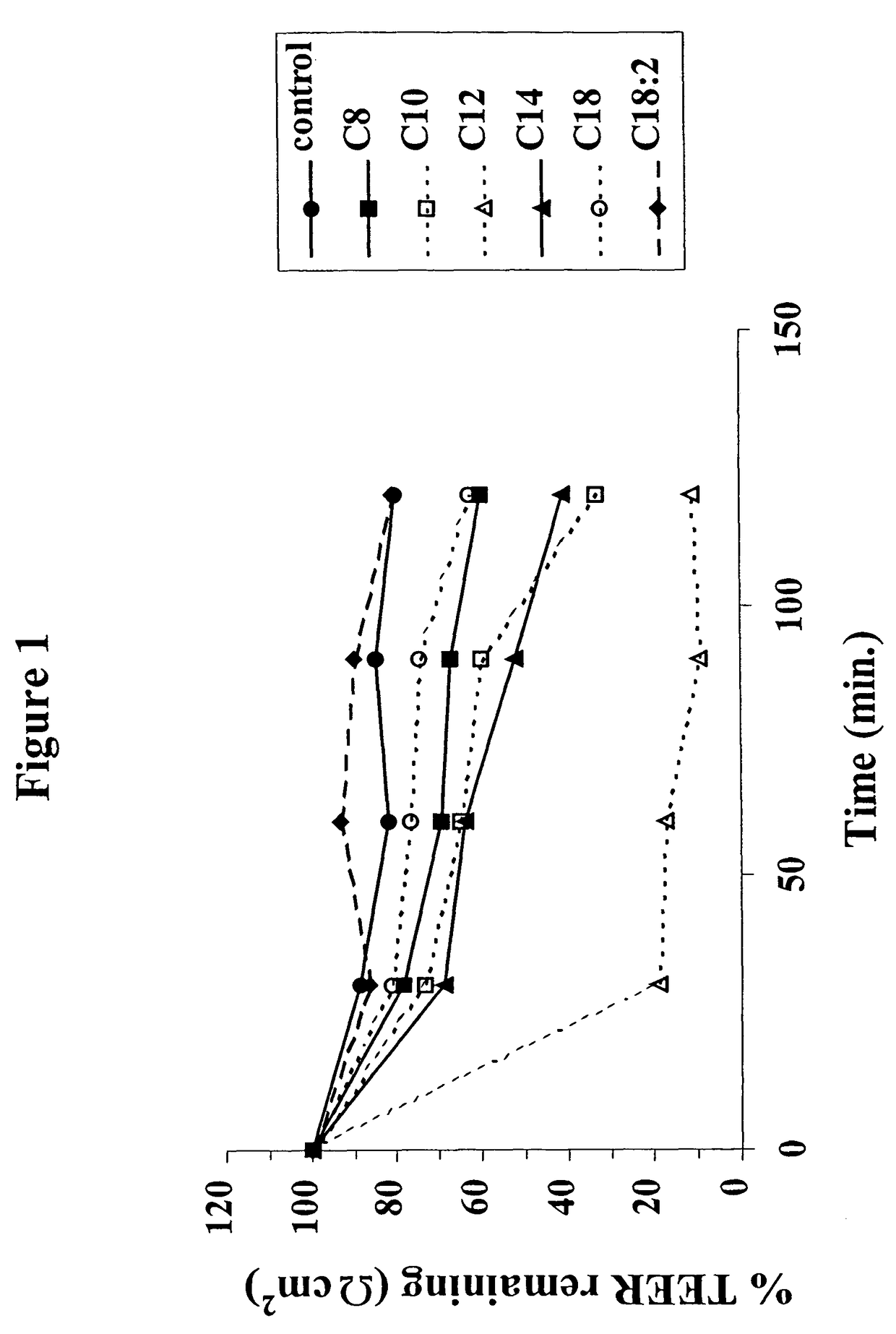
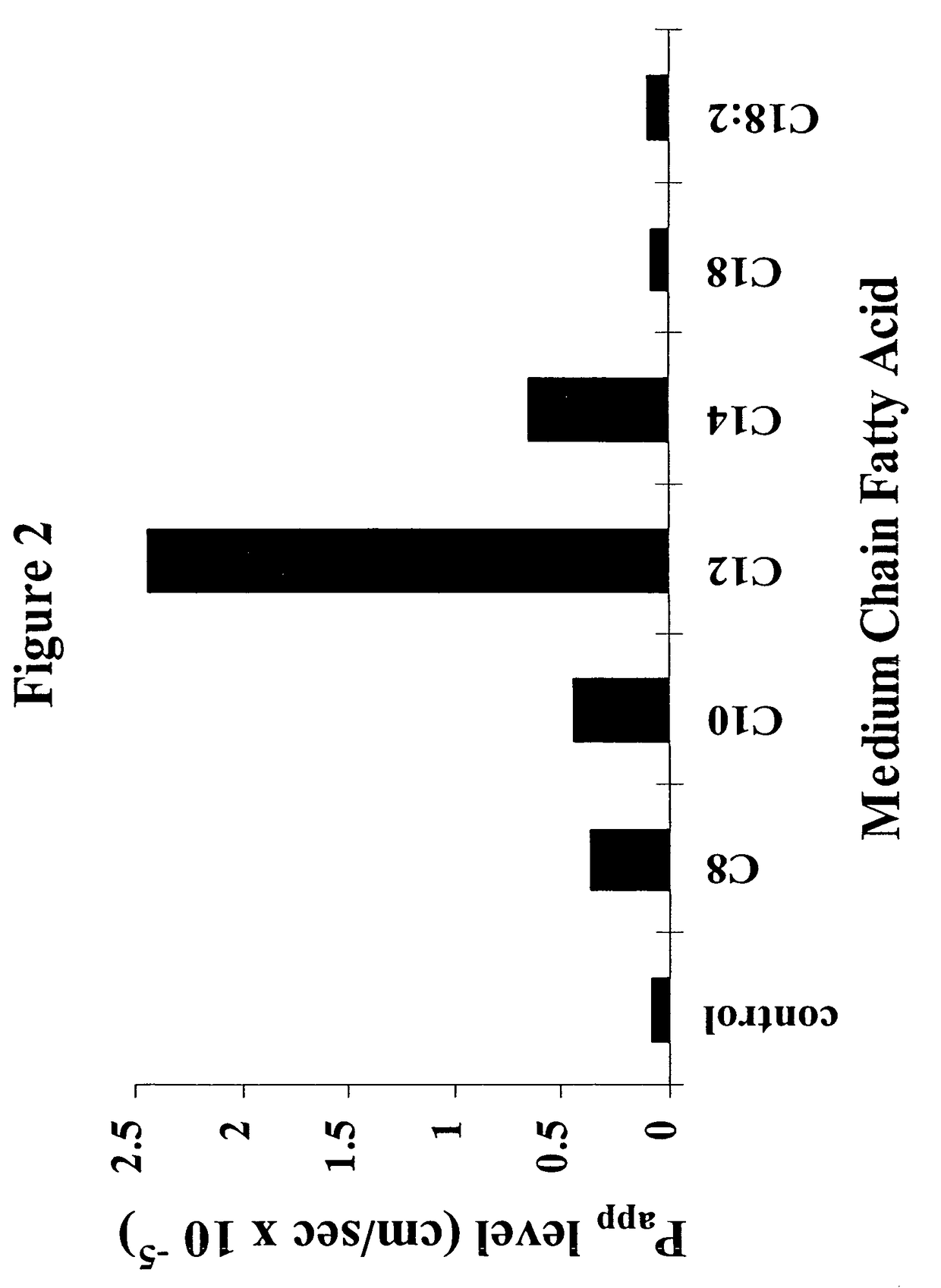
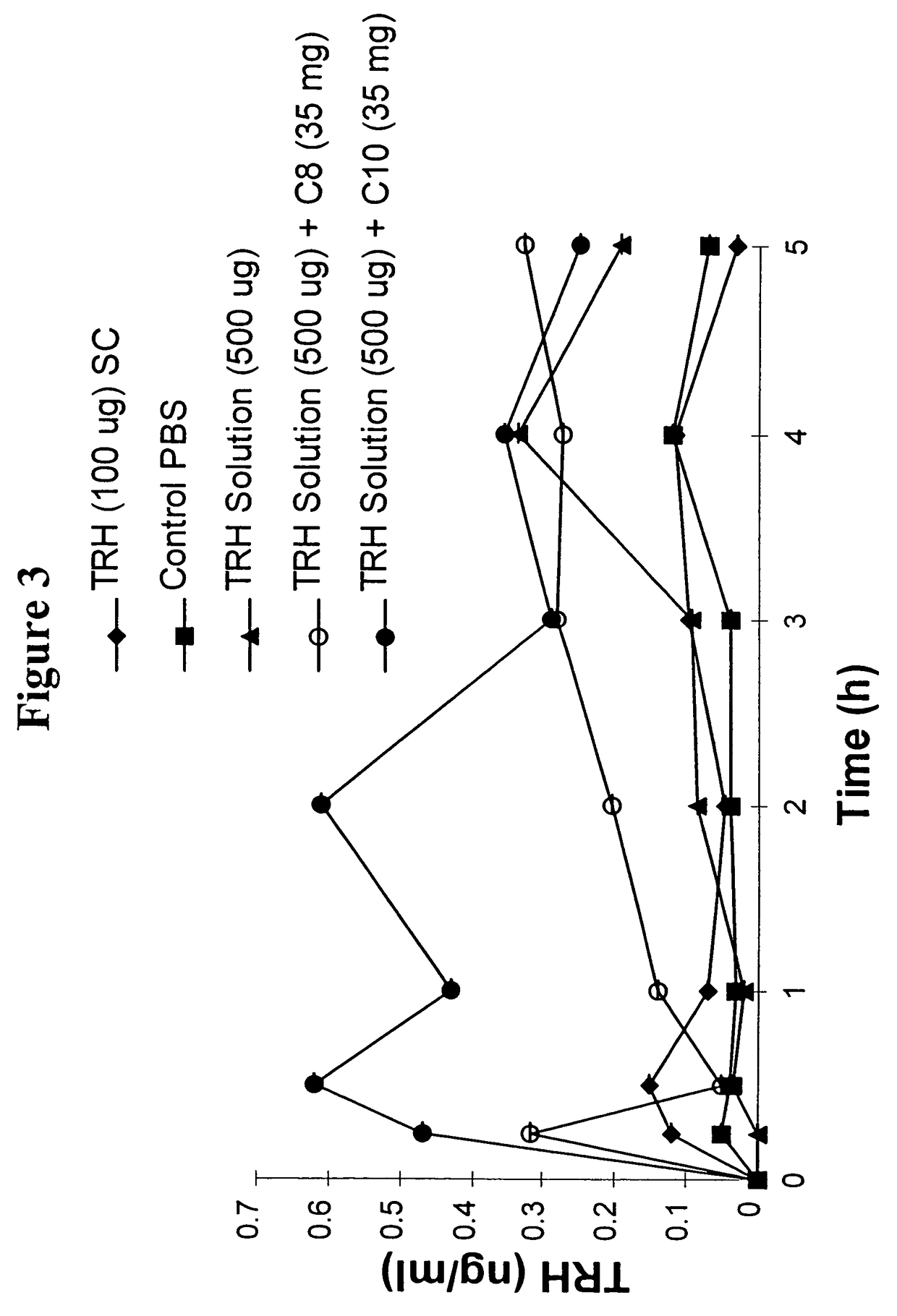
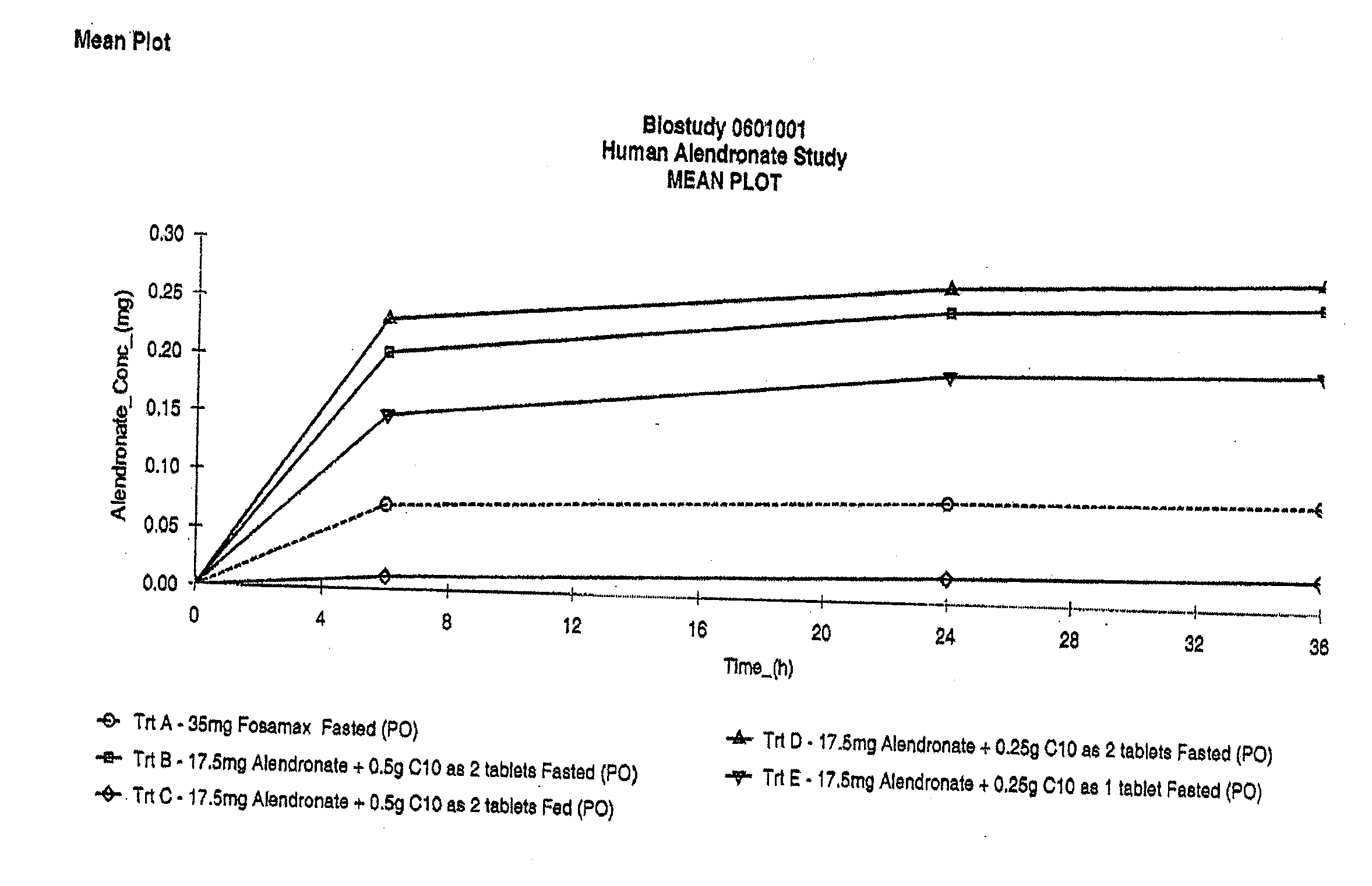
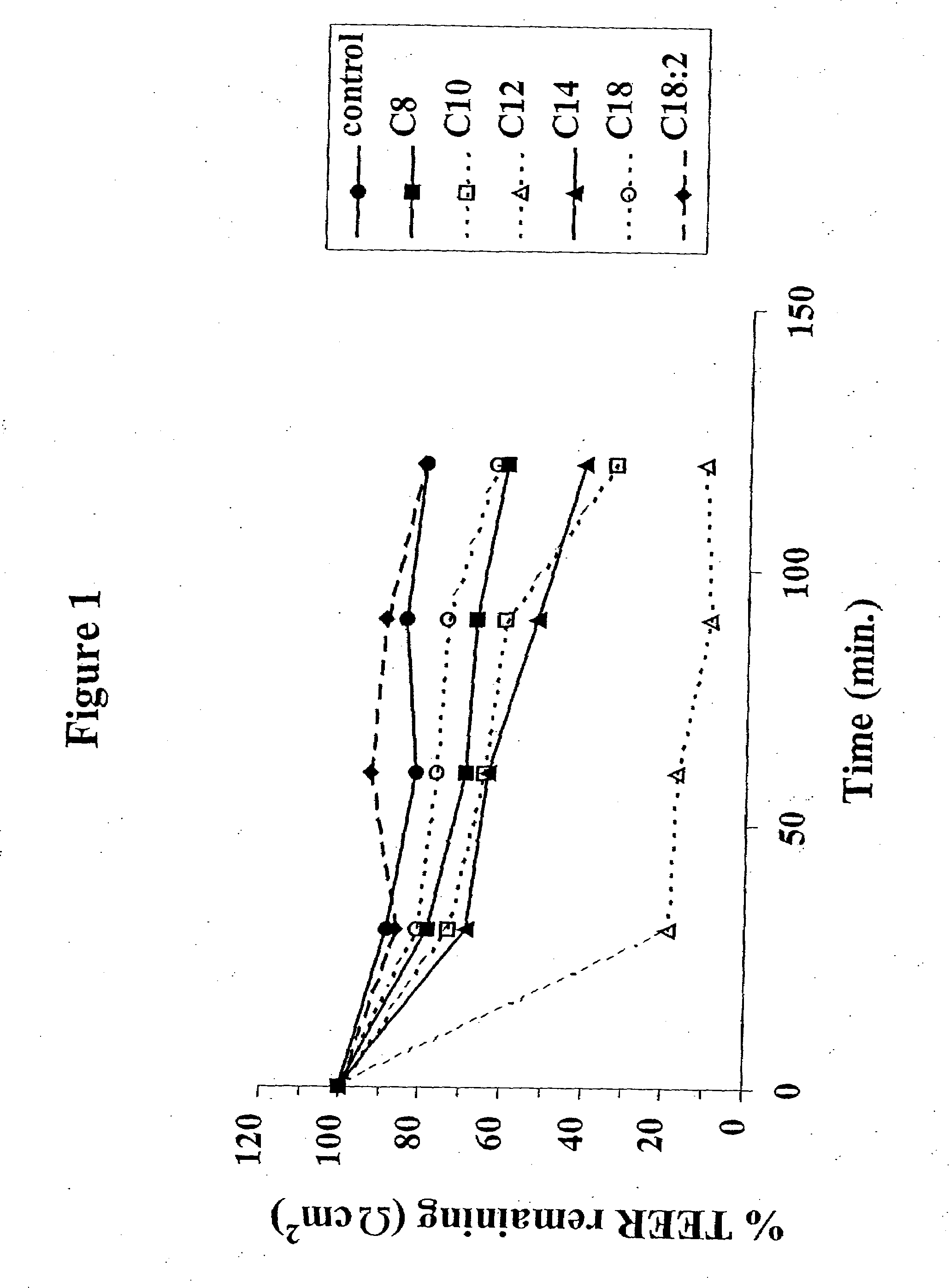
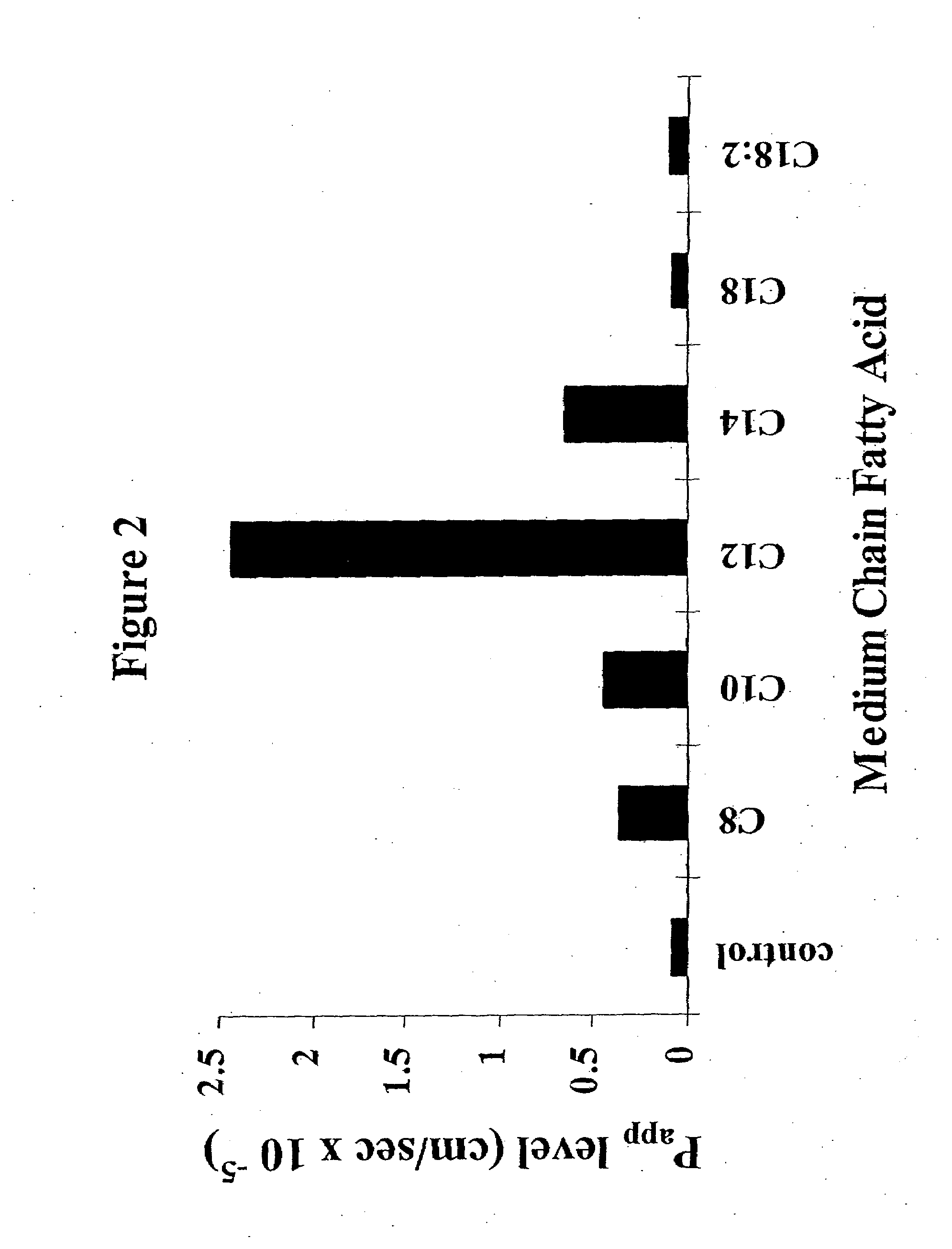
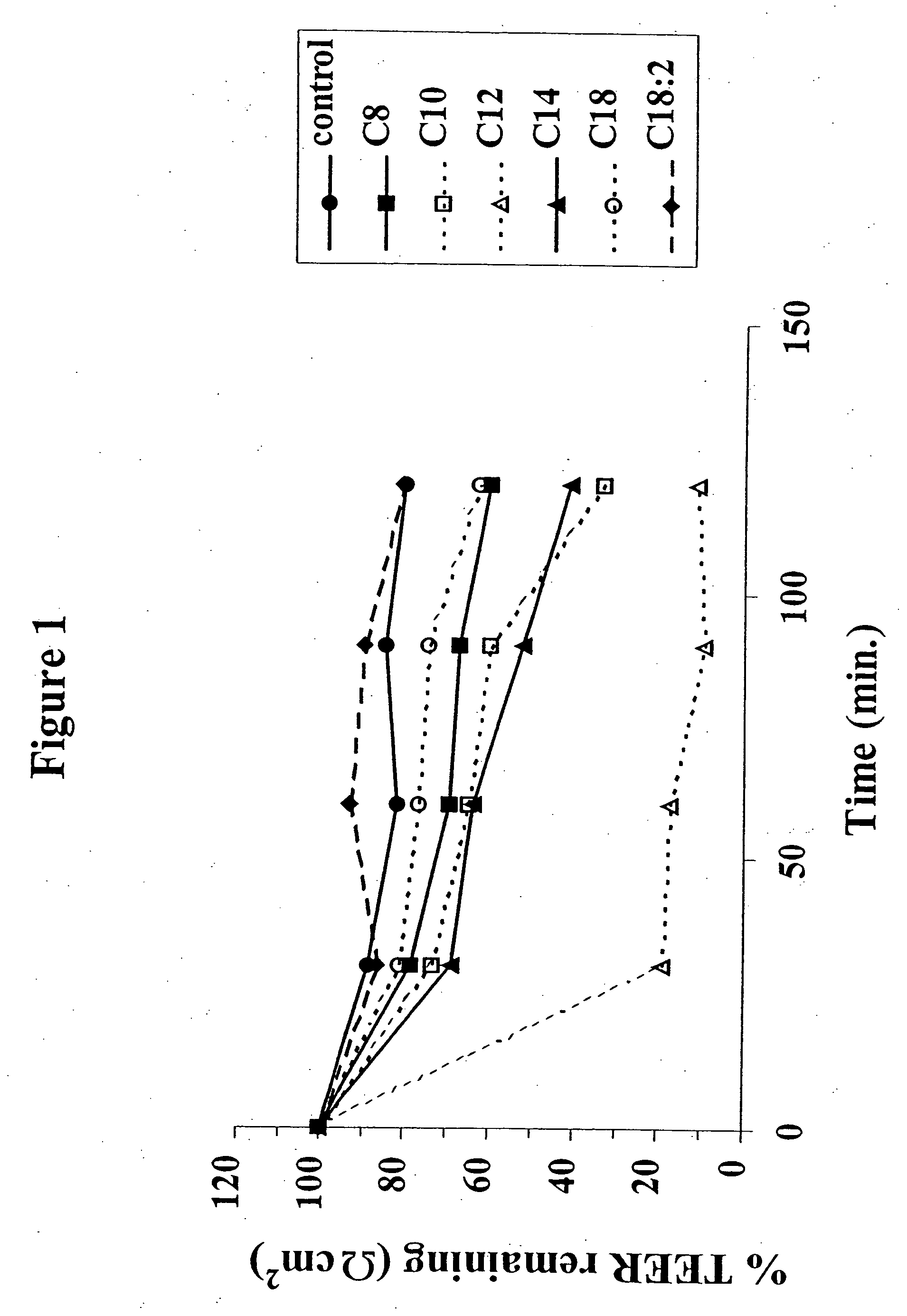
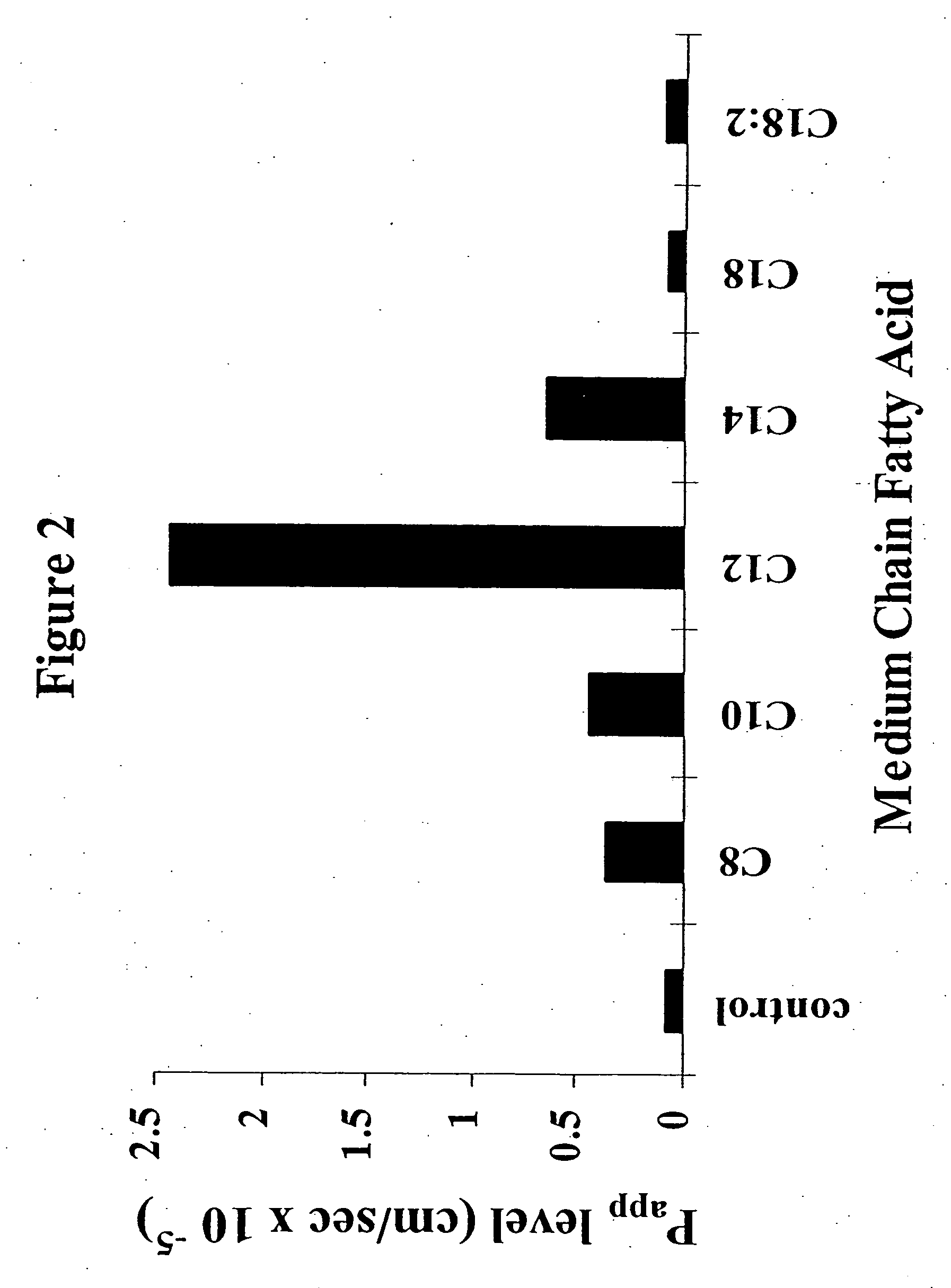
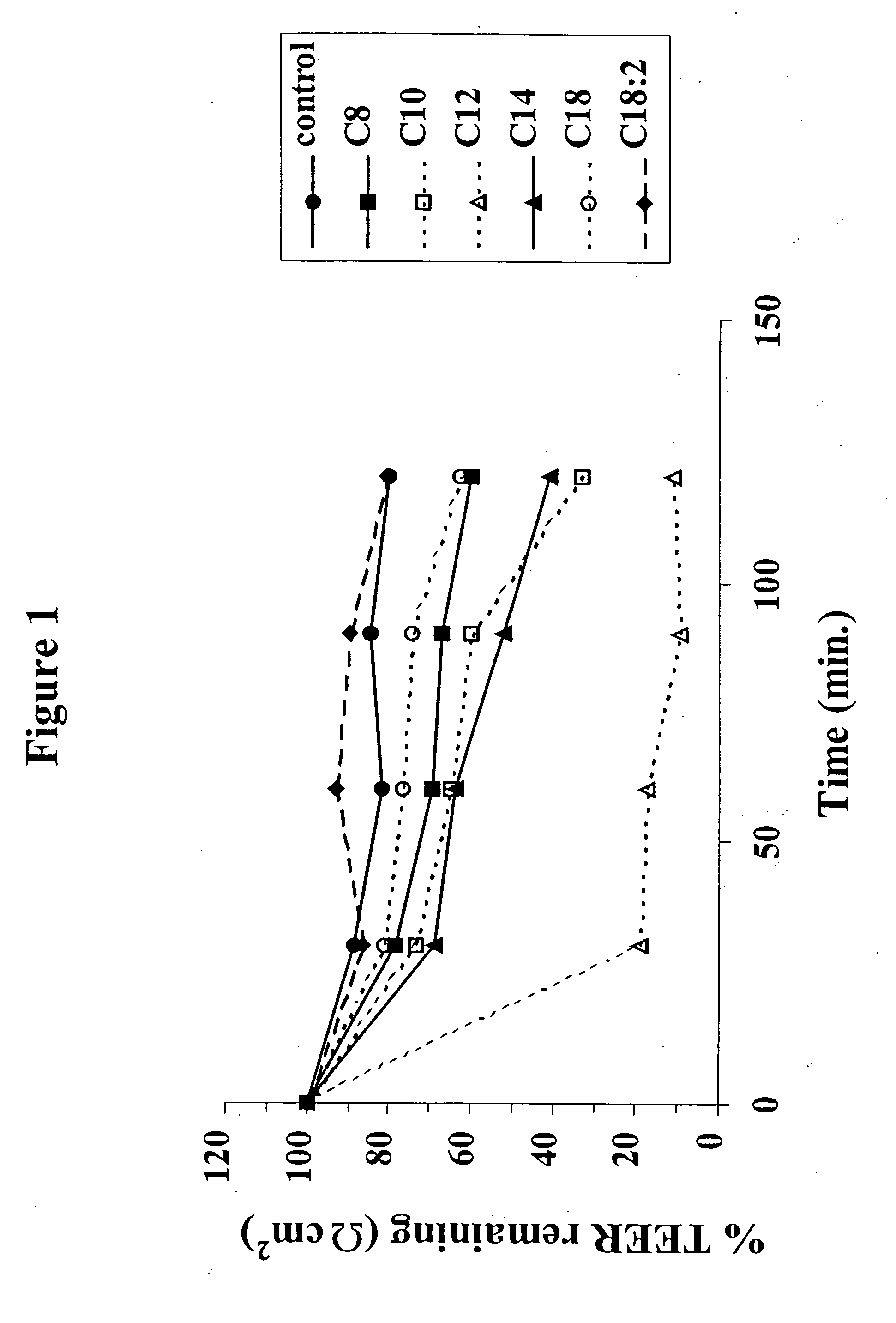
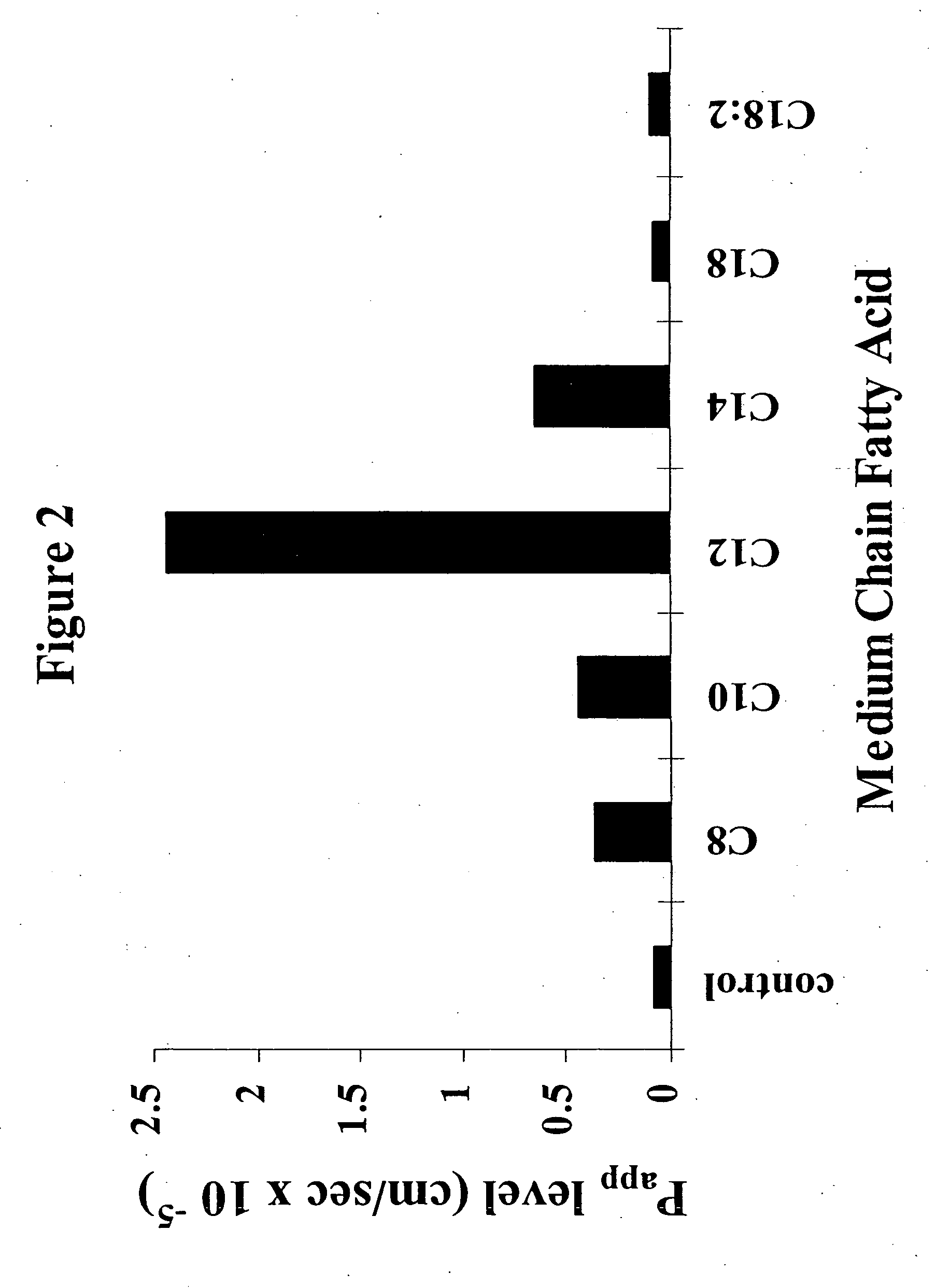
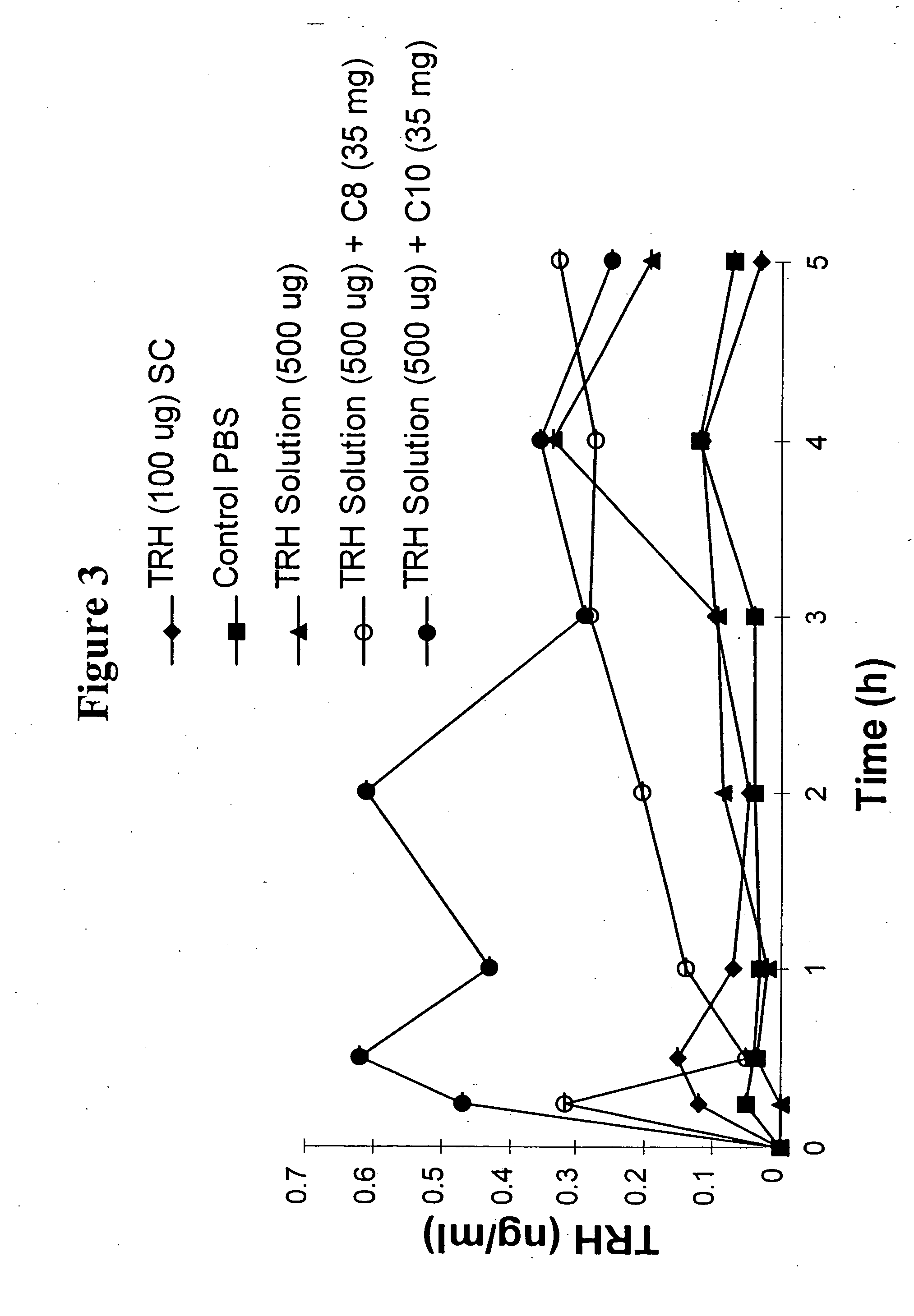
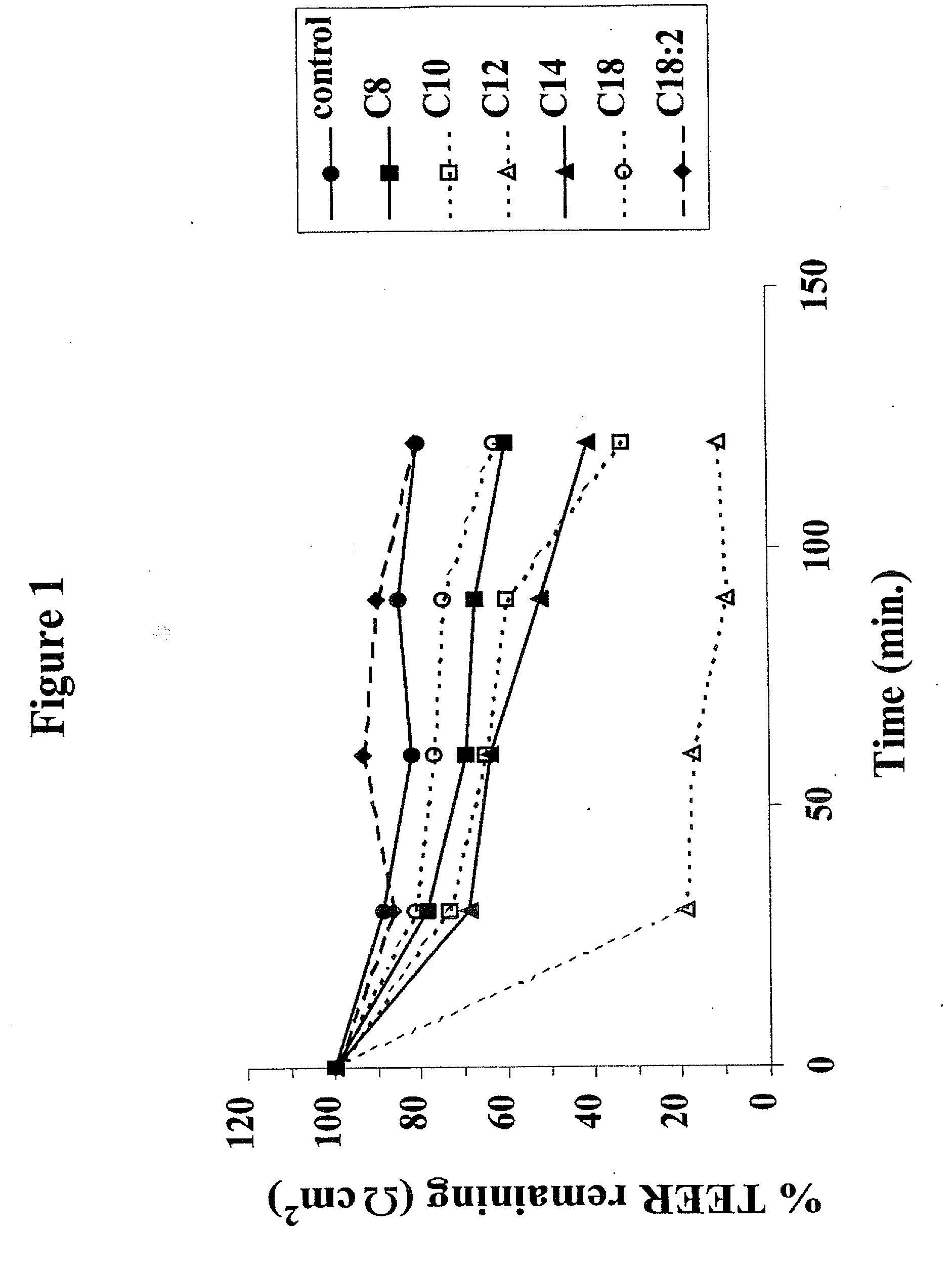
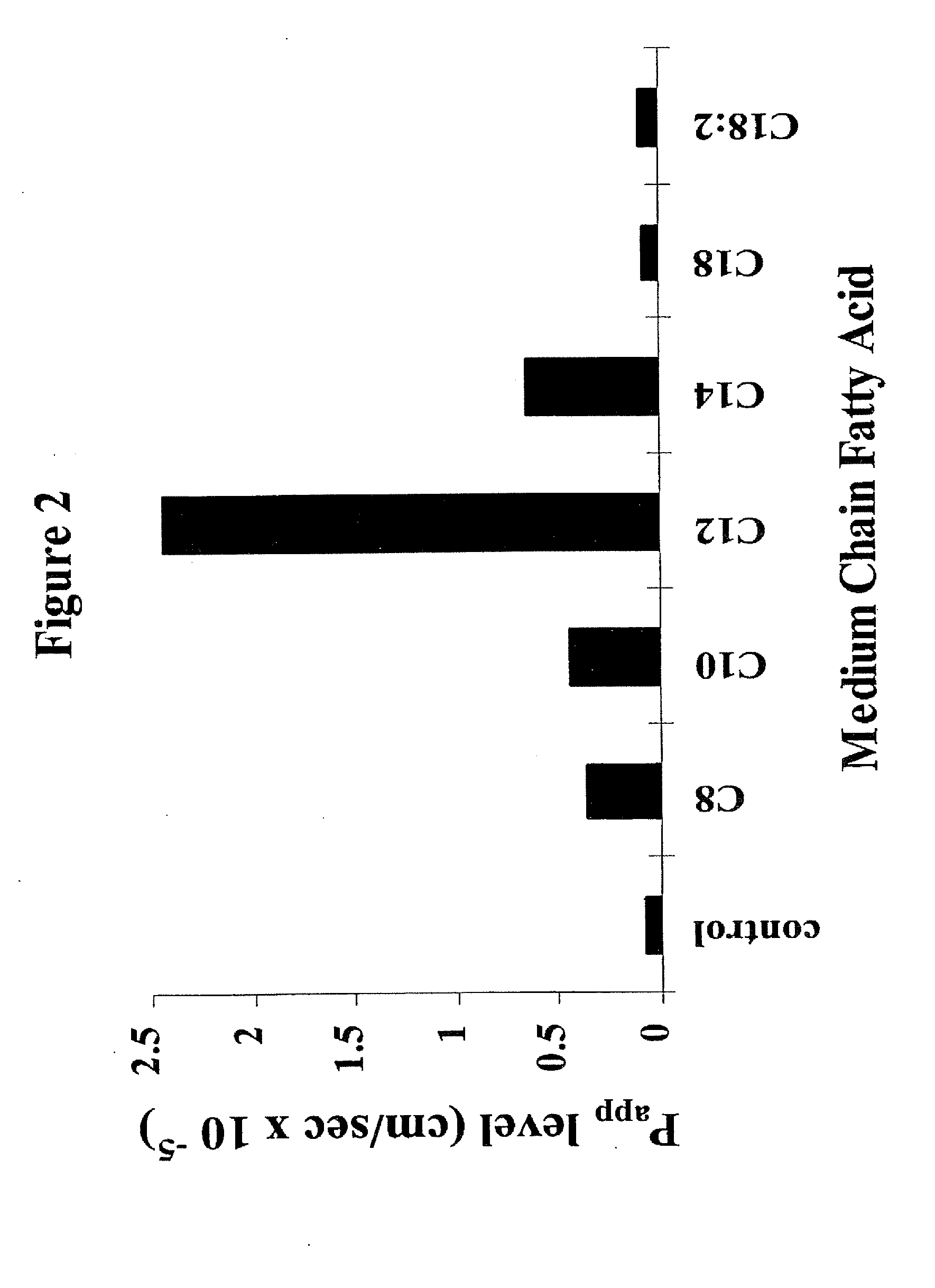
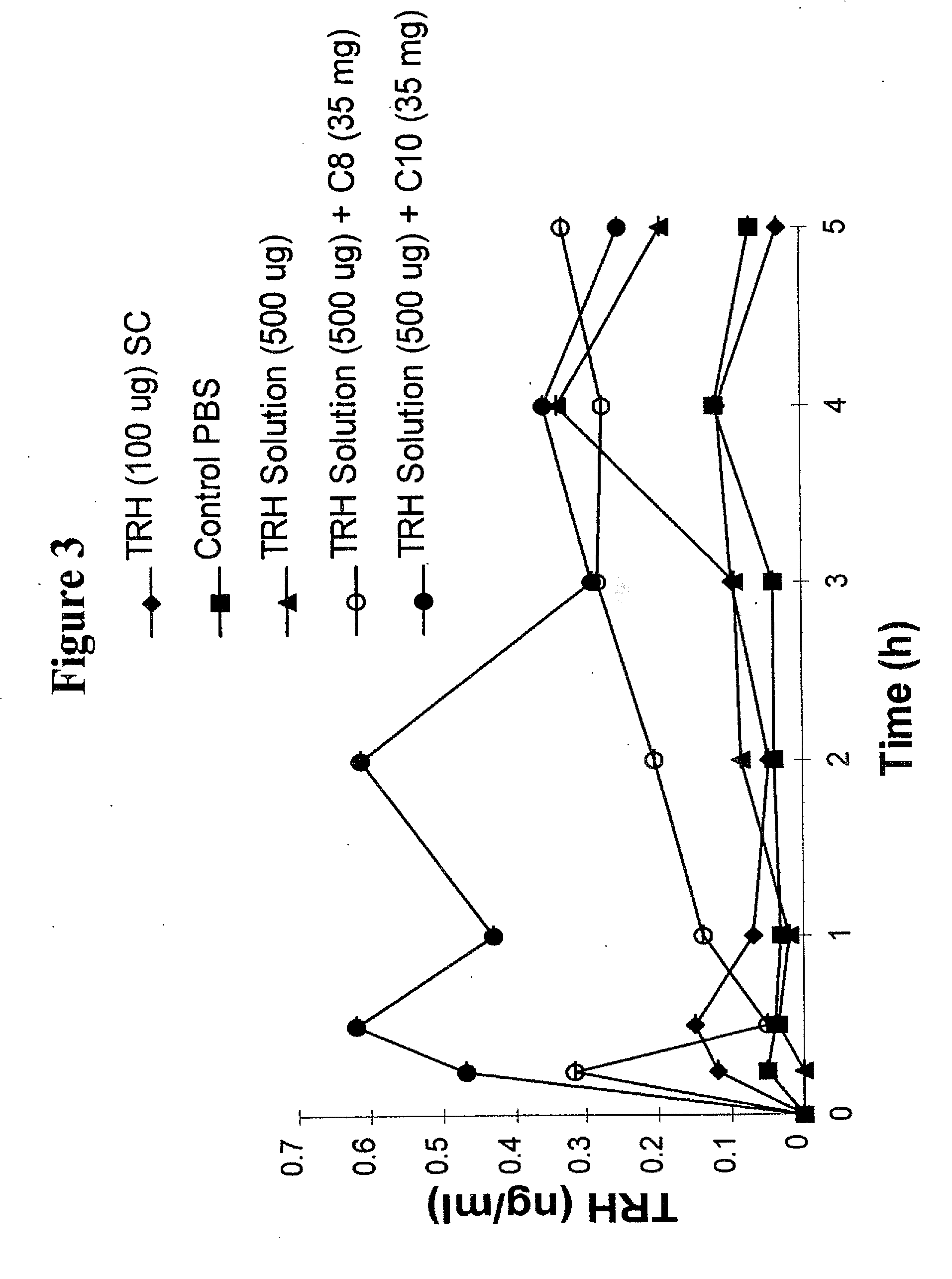
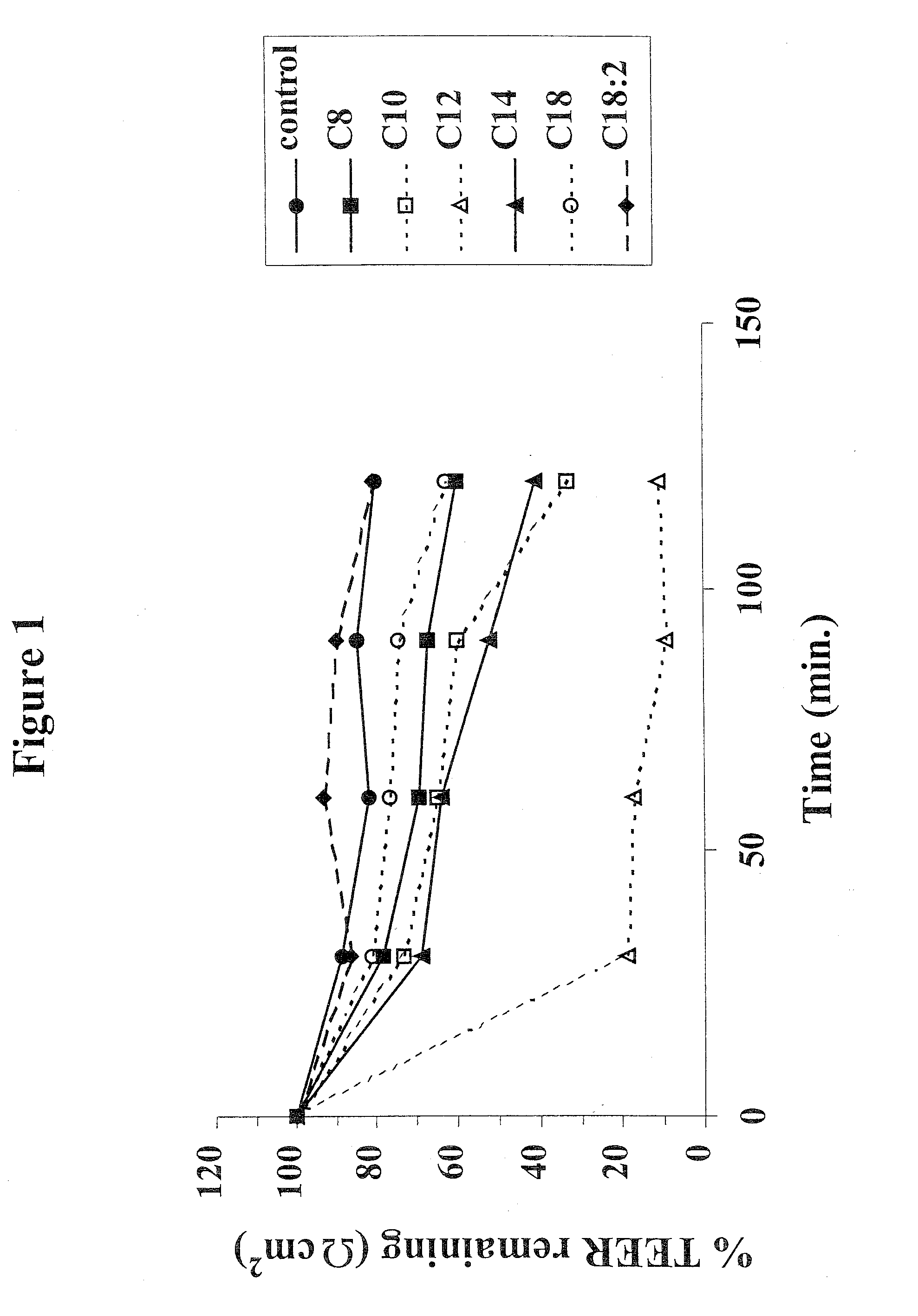
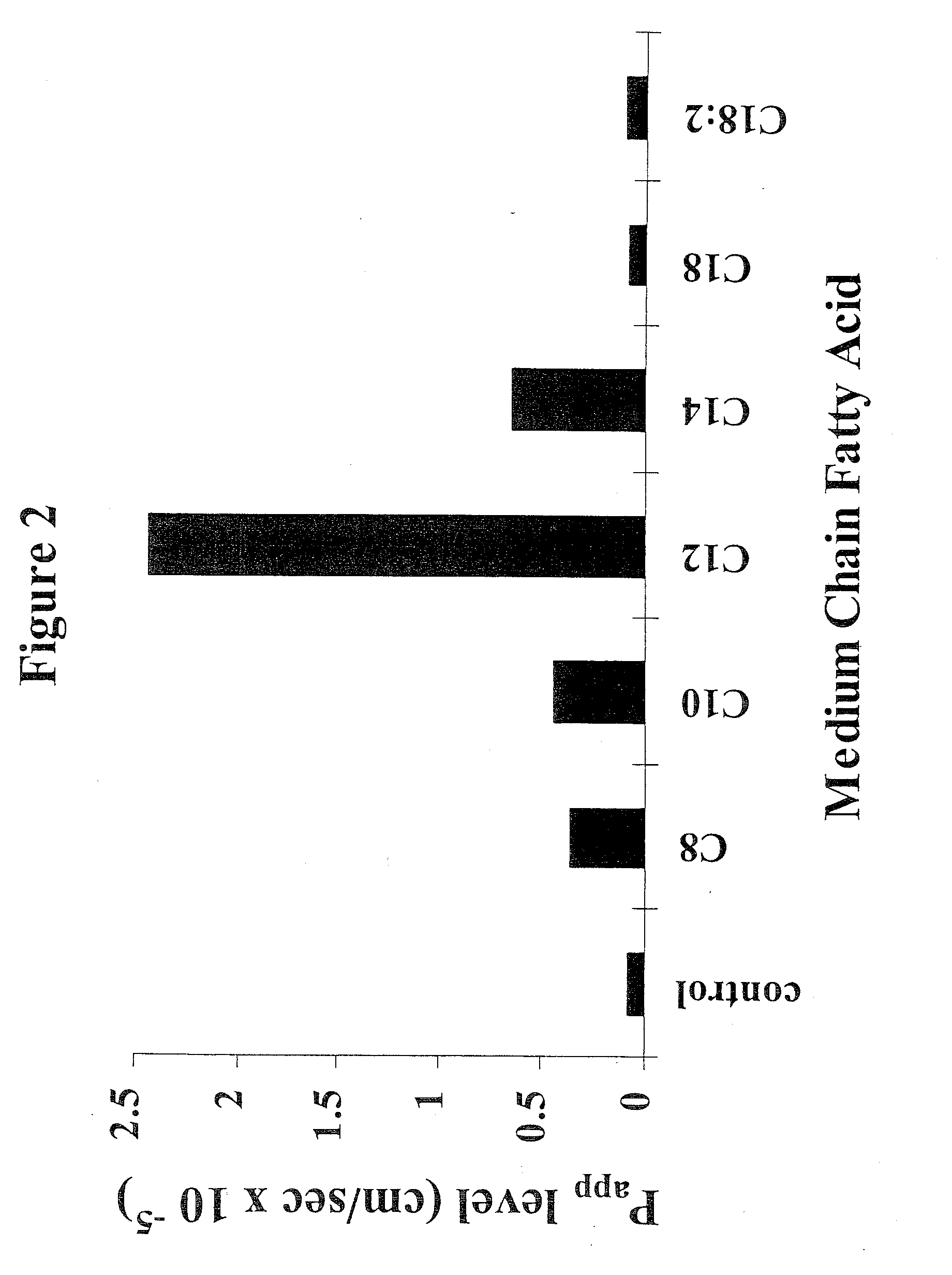
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20070238707A1Improve oral bioavailabilityMinimizes risk of local irritationBiocideAntipyreticDelayed Release Dosage FormDiphosphonates
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to enhance intestinal delivery of the bisphosphonate to the underlying circulation. Preferably, the enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms, and the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
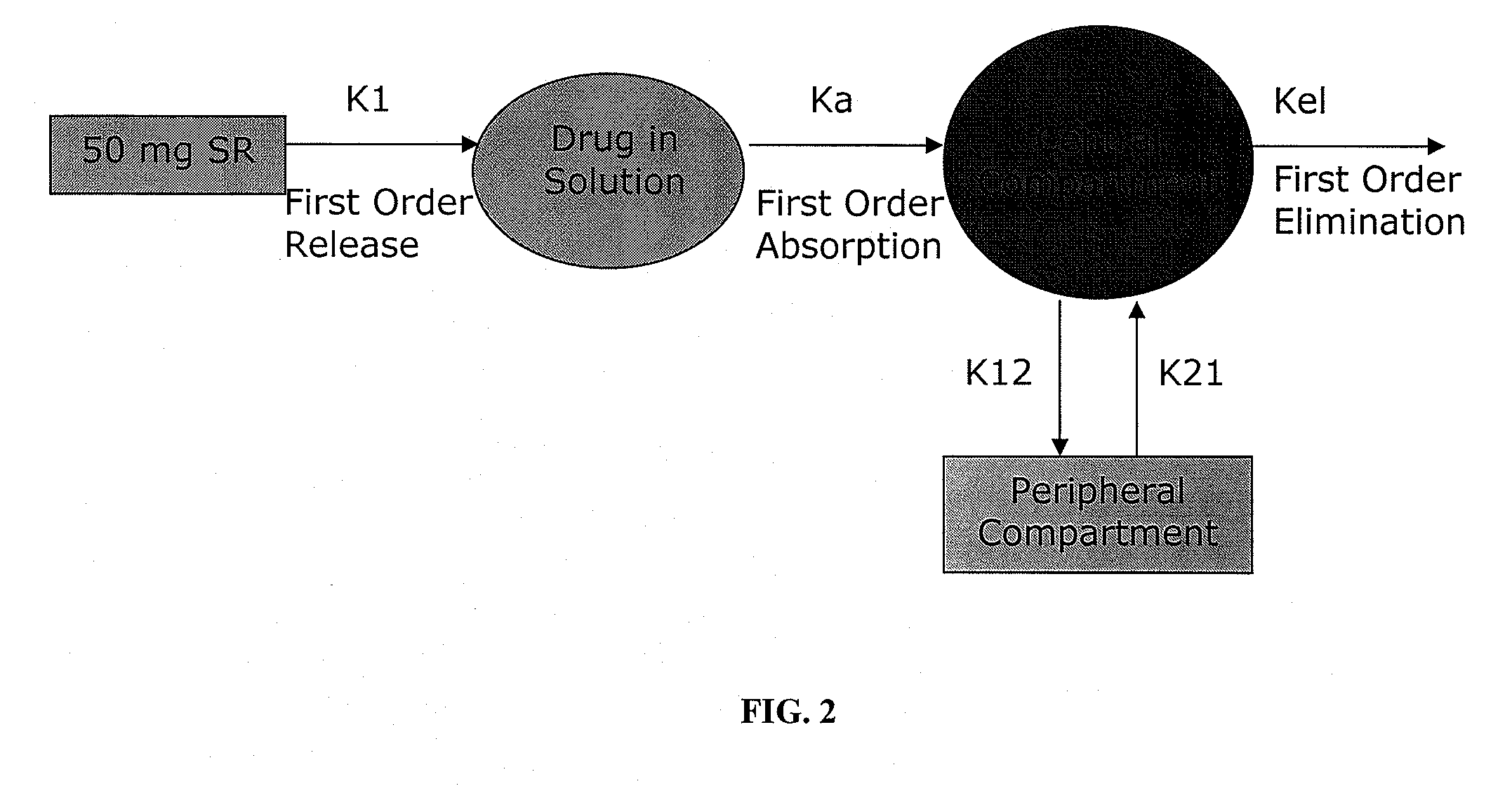
Formulation for sustained delivery
Disclosed is an extended or controlled release dosage form of citalopram or its related forms and other newer antidepressants for oral administration to treat chronic patients suffering from depression and to minimize the side effects associated with the current drug treatment.
Owner:CHALLAPALLI PRASAD V N +2
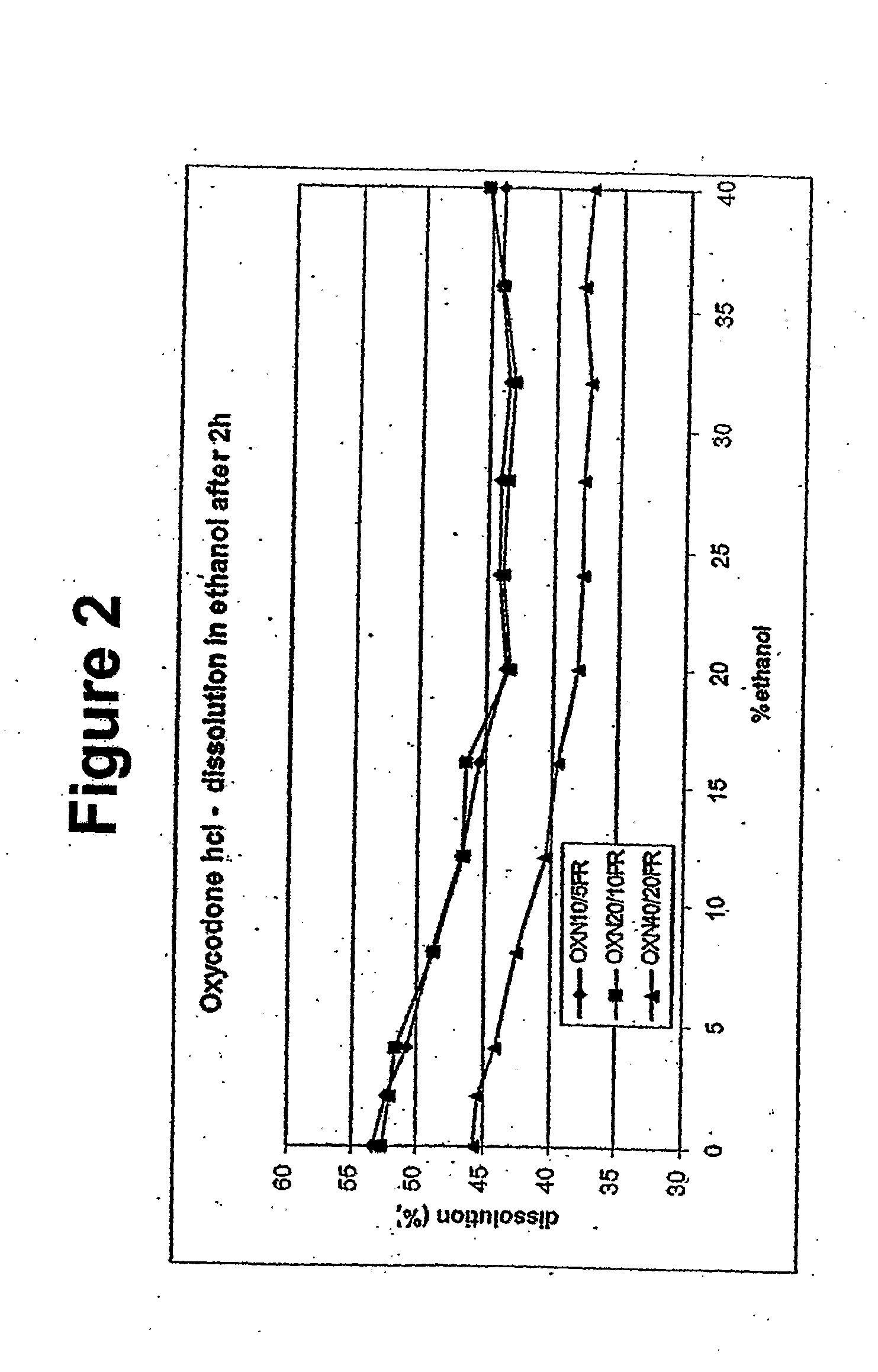
Alcohol Resistant Dosage Forms
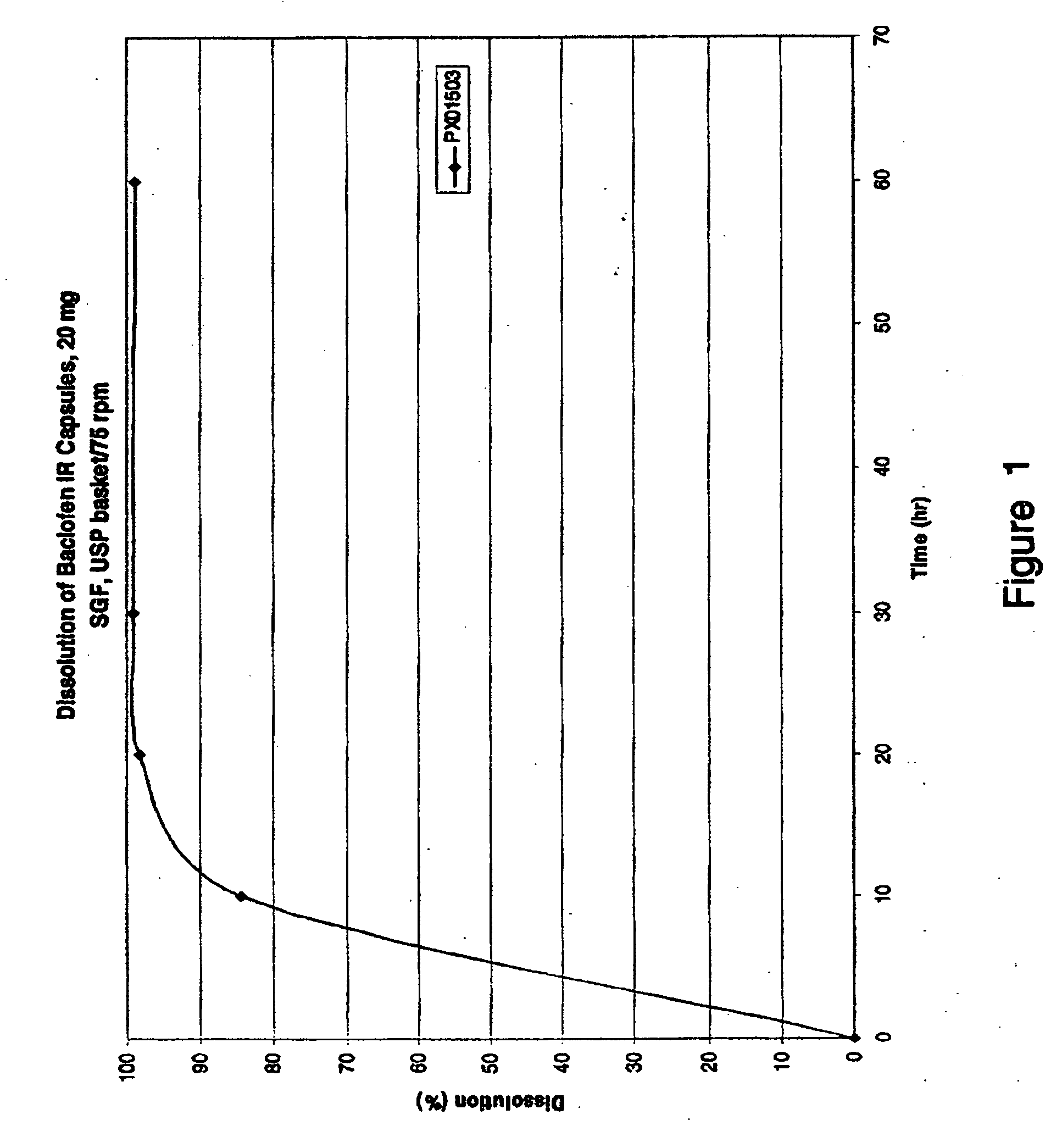
Disclosed in certain embodiments is a controlled release dosage form comprising a matrix comprising a pharmaceutically acceptable salt of an opioid analgesic in a controlled release material; wherein less than 25% of the opioid salt is released after 1 hour of in-vitro dissolution of the dosage form in 900 ml of Simulated Gastric Fluid with 20% ethanol using a USP Apparatus I (basket) apparatus at 100 rpm at 37 degrees C.°.
Owner:PURDUE PHARMA LP
System for manufacturing controlled release dosage forms, such as a zero-order release profile dosage form manufactured by three-dimensional printing
ActiveUS7300668B2Additive manufacturing apparatusInorganic non-active ingredientsHomogeneous distributionTape Dosage Form
The present invention includes controlled release dosage forms and methods of designing and manufacturing dosage forms to obtain specific release profiles, for example, zero-order release profiles, escalating release profiles or decreasing release profiles. The dosage forms of the present invention can include spatial variation of API concentration in the dosage form and can include nested regions. Dosage forms according to the present invention may be manufactured by any appropriate method for obtaining the internal structure as disclosed herein for producing zero-order release profiles and increasing or decreasing release profiles. The invention further includes methods of manufacturing such dosage forms, such as by three-dimensional printing, possibly also including compression of the dosage form after three-dimensional printing. The invention further includes methods of designing such dosage forms. Release profiles from non-uniform distributions of API concentration may be predicted based on simple experiments with uniform-concentration dosage forms.
Owner:MASSACHUSETTS INST OF TECH +1
Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer
InactiveUS6316031B1Reduce reunionStable productionLiquid surface applicatorsGranular deliveryHydrophobic polymerDissolution
A stabilized solid controlled release dosage form having a coating derived from an aqueous dispersion of ethylcellulose is obtained by overcoating a substrate including a therapeutically active with an aqueous dispersion of ethylcellulose and then curing the coated substrate at a temperature and relative humidity elevated to a suitable level above ambient conditions until the coated dosage form attains a stabilized dissolution profile substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:PURDUE PHARMA LP
Solid oral dosage form containing an enhancer
InactiveUS20070148228A1BiocideCyclic peptide ingredientsDelayed Release Dosage FormPharmaceutical drug
The invention relates to a pharmaceutical composition and oral dosage forms comprising an HDAC inhibitor in combination with an enhancer to promote absorption of the HDAC inhibitor at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:MERRION RES I
Solid Oral Dosage Form Containing an Enhancer
The invention relates to a pharmaceutical composition, particularly oral dosage forms, comprising a DAC inhibitor in combination with an enhancer to promote absorption of the DAC inhibitor at the GIT cell lining. The enhancer is a medium chain fatty acid or derivative thereof having a carbon chain length of from 6 to 20 carbon atoms. In certain embodiments, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:MERRION RES I
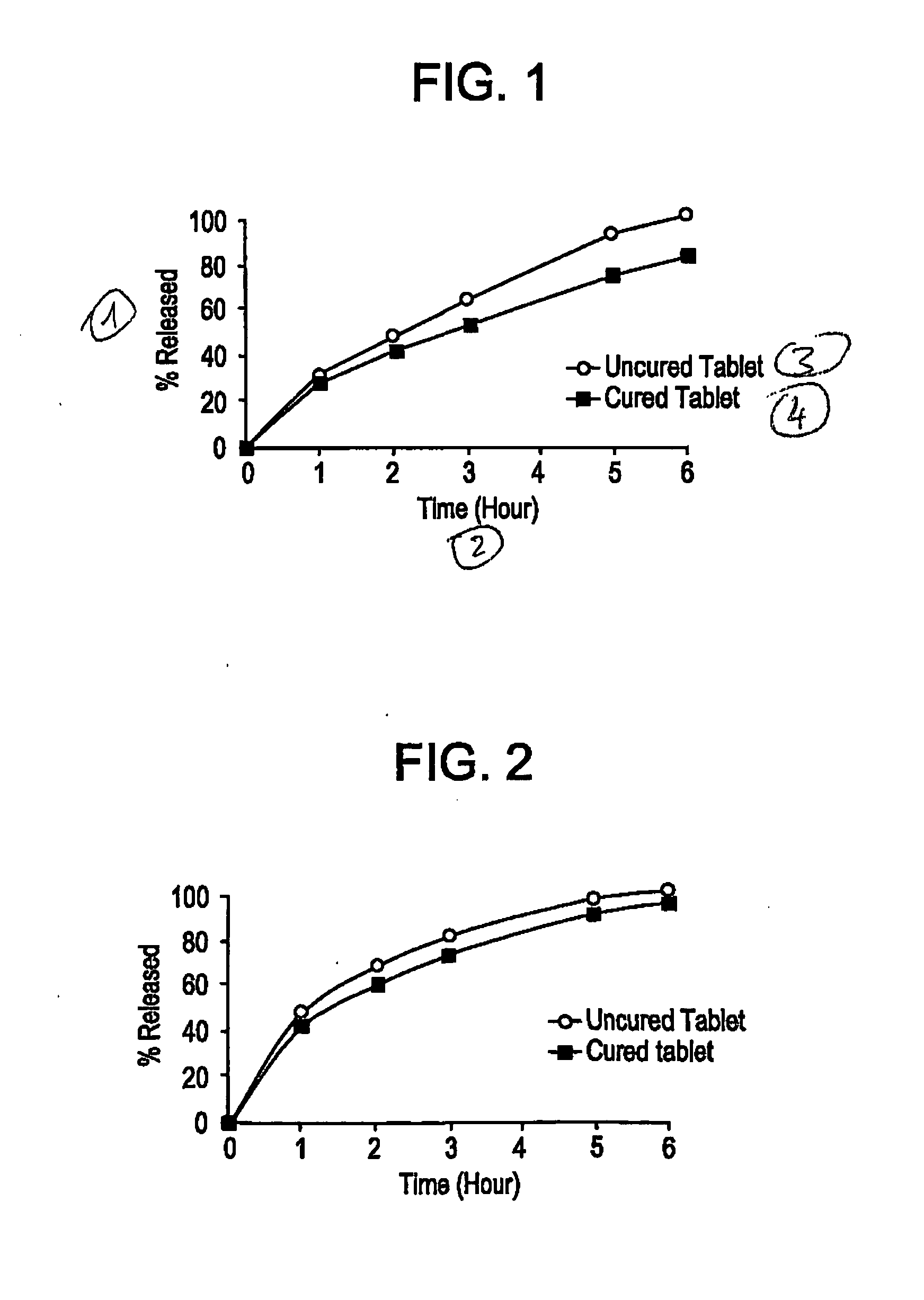
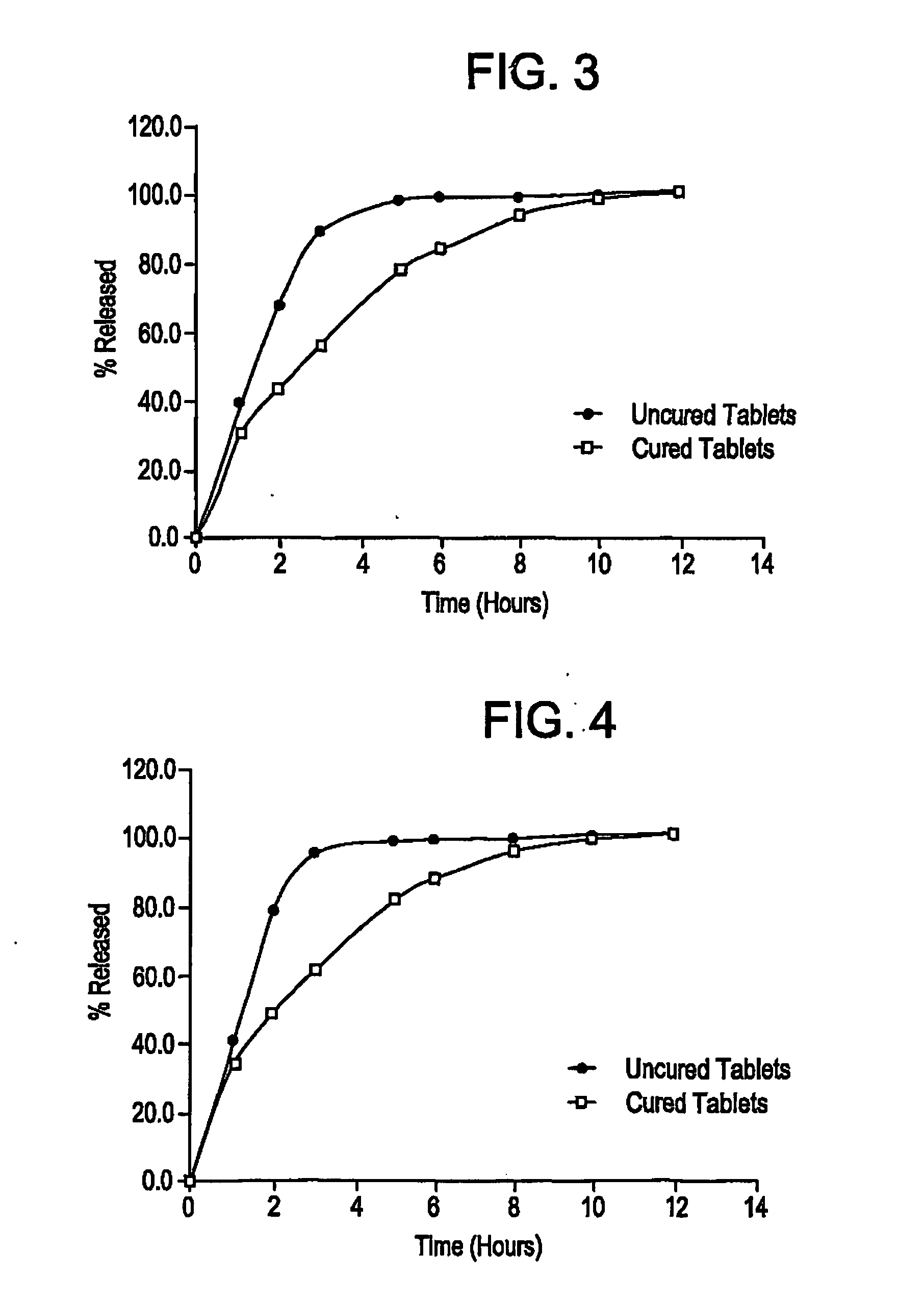
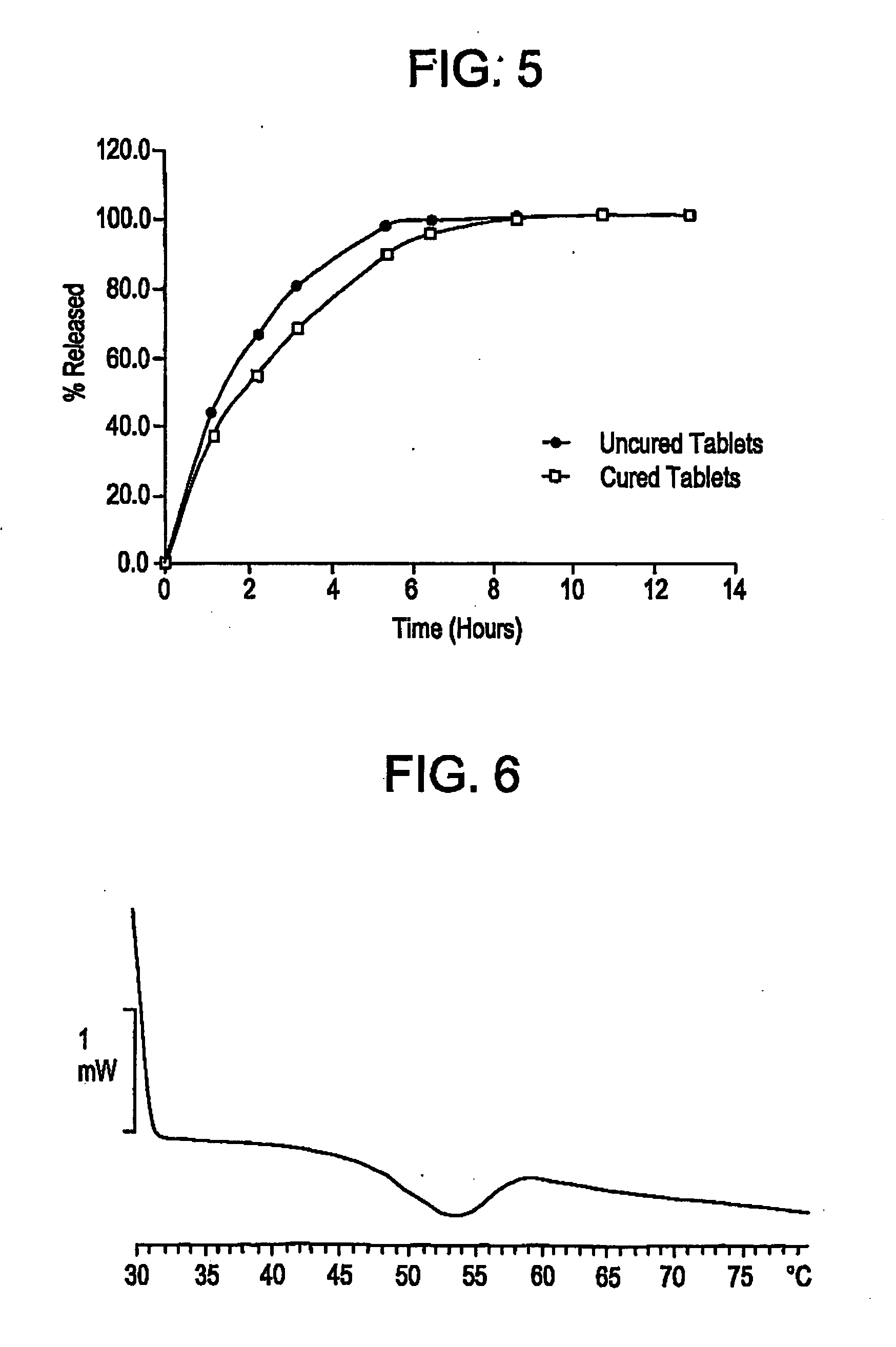
Controlled release dosage forms using acrylic polymer, and process for making
Process for dry mixing a controlled release oral dosage form are provided. The dosage form is produced by mixing, tableting, and curing dosage forms. The cured dosage forms exhibit controlled release properties superior to those of uncured tablets.
Owner:ENDO PHARMA INC
Pharmaceutical dosage forms having immediate release and/or controlled release properties
ActiveUS20060057197A1Prolonged in vivo absorptionPromote absorptionPowder deliveryPharmaceutical non-active ingredientsControl releaseMedicine
The present invention relates generally to pharmaceutical dosage forms comprising: an absorption window active agent; a controlled release component comprising enteric-coated controlled release beads, wherein the enteric-coated release beads comprise at least two pH-sensitive polymer layers. The controlled-release dosage forms provide good bioavailability of absorption window active agents.
Owner:IMPAX LAB LLC
Solid oral dosage form containing an enhancer
InactiveUS20070196464A1Improve oral bioavailabilityMinimizes risk of local irritationBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
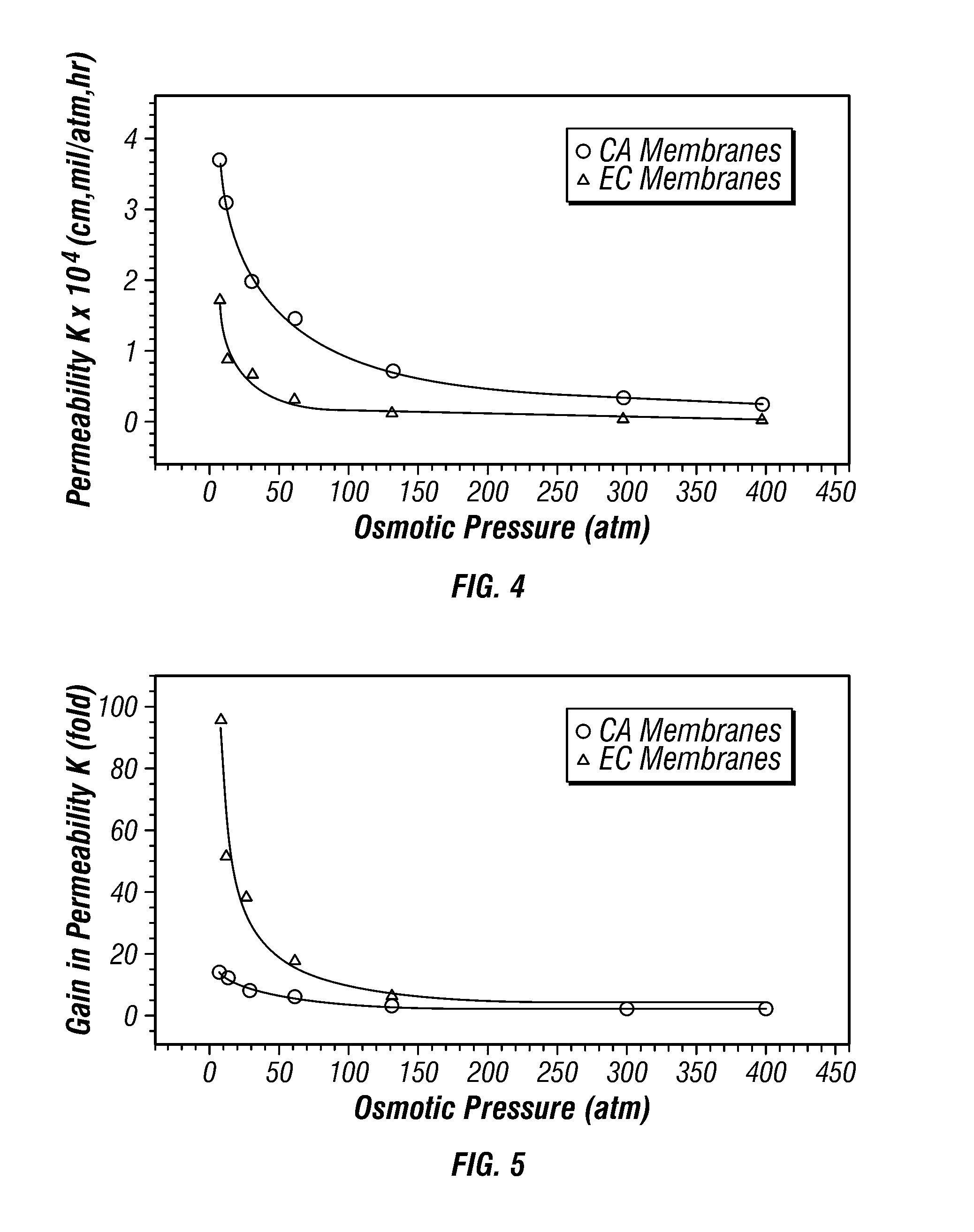
Extended Release Dosage Form
InactiveUS20070128279A1Satisfies needEnhances fluid fluxBiocideAntipyreticOsmotic pumpBiomedical engineering
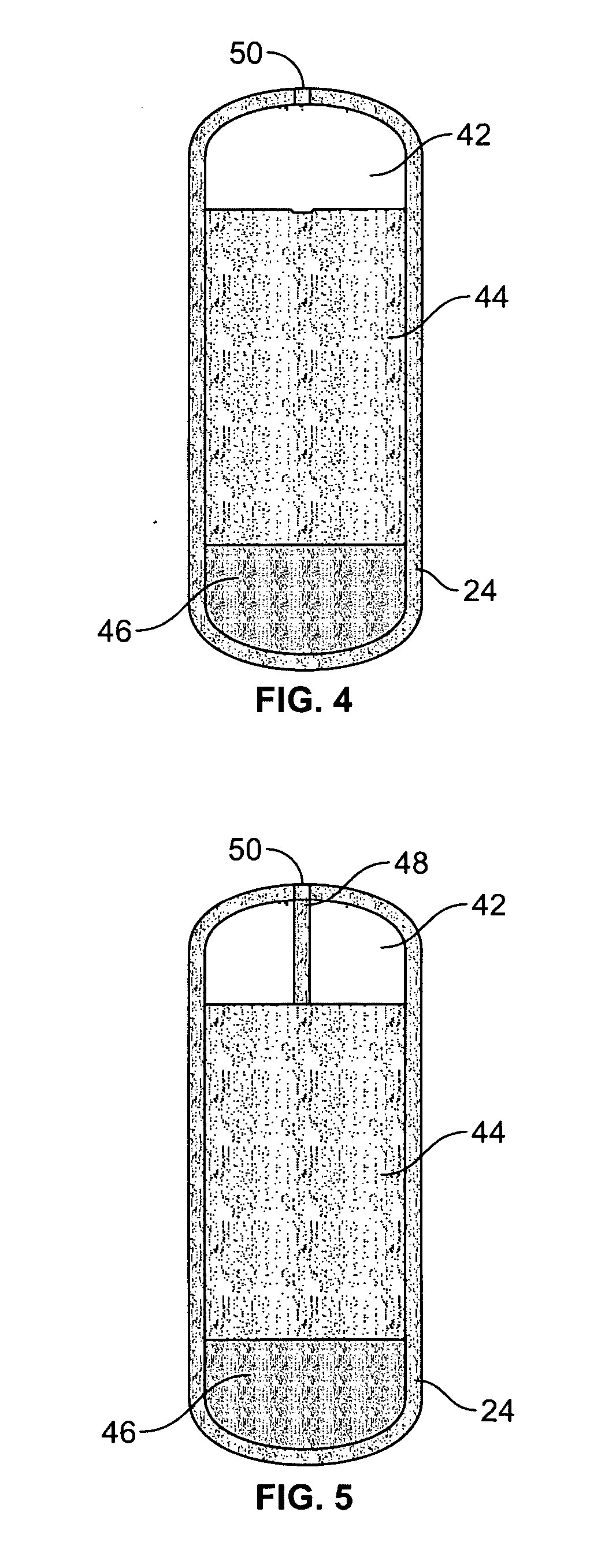
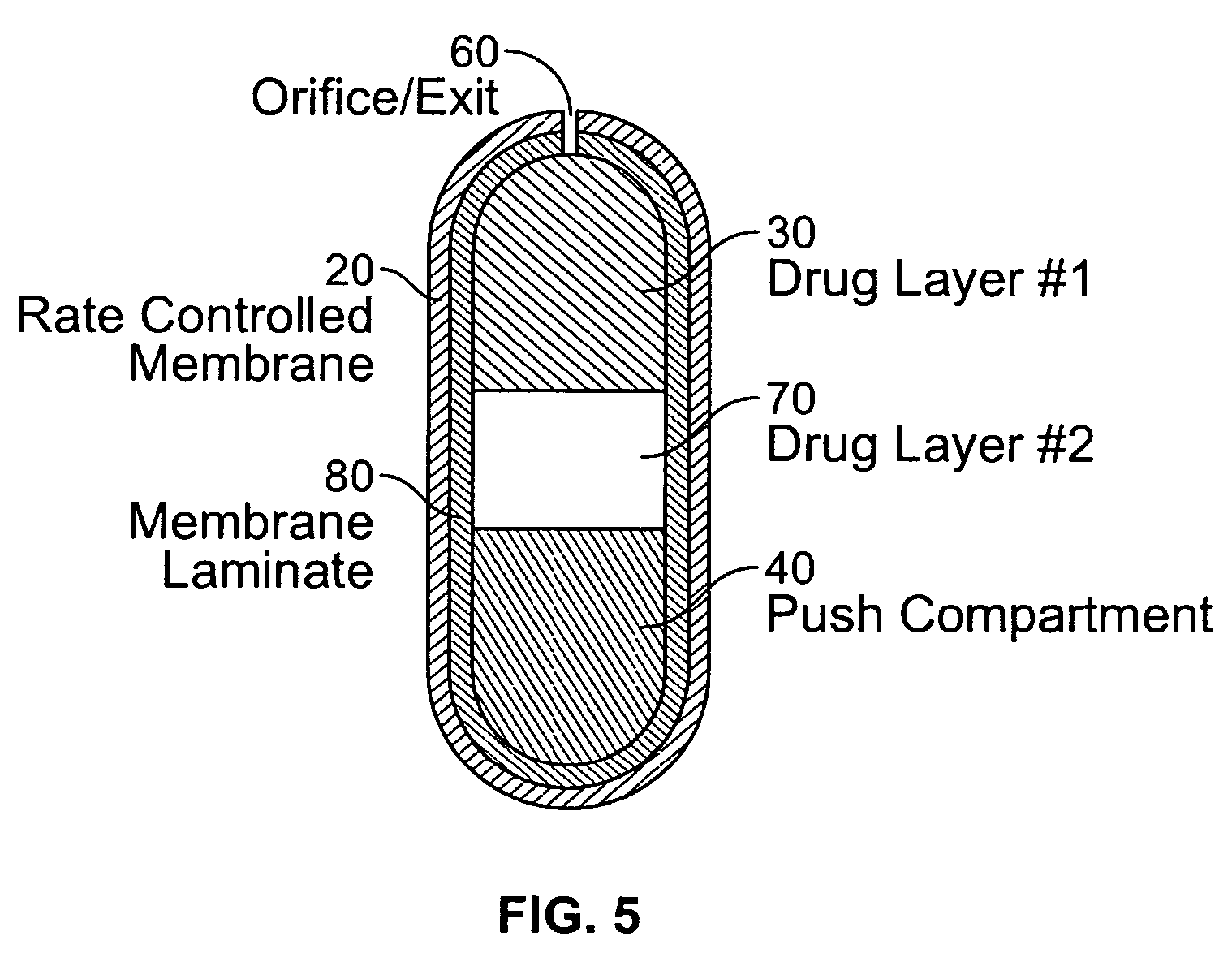
A membrane system comprising an interior wall, a fluid-permeable exterior wall surrounding the interior wall and an internal compartment defined by the membrane system, wherein fluid permeability of the interior wall is responsive to osmolarity of an osmotic core within the internal compartment are disclosed. A controlled release dosage form comprising the membrane system and a process for delivering an osmotically active formulation from an osmotic pump over an extended period of time are also disclosed.
Owner:ALZA CORP
Solid oral dosage form containing an enhancer
InactiveUS20080275001A1BiocideOrganic active ingredientsDelayed Release Dosage FormAdditive ingredient
The invention relates to a solid oral dosage form comprising a pharmaceutically active ingredient in combination with an enhancer which enhances the bioavailability and / or the absorption of the active ingredient. Accordingly, a solid oral dosage form comprises a drug and an enhancer wherein the enhancer is a medium chain fatty acid ester, ether or salt or a derivative of a medium chain fatty acid, which is, preferably, solid at room temperature and which has a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Rosiglitazone formulations
Rosiglitazone is a drug used to treat type 2 diabetes. Methods for the formation of amorphous rosiglitazone and formulations comprising the amorphous rosiglitazone are described. Other formulations include pulsed-release formulations and formulations for retention in the stomach and upper gastrointestinal tract. Controlled-release dosage form include those wherein the maximum plasma concentration of rosiglitazone occurs greater than one hour after administration to a human and / or wherein less than 75 percent by weight of the rosiglitazone is released at 1 hour after immersion in simulated gastric fluid.
Owner:ACTAVIS GRP PTC EHF
Controlled release dosage forms combining immediate release and sustainted release of low-solubility drug
InactiveUS20080299188A1Short biological half-lifePromote absorptionPowder deliveryOrganic active ingredientsSolubilityImmediate release
A controlled release dosage form comprises an immediate release portion and an enteric coated sustained release core.
Owner:PFIZER INC
Controlled release dosage forms
InactiveUS20060233880A1Organic active ingredientsPeptide/protein ingredientsOral medicationBULK ACTIVE INGREDIENT
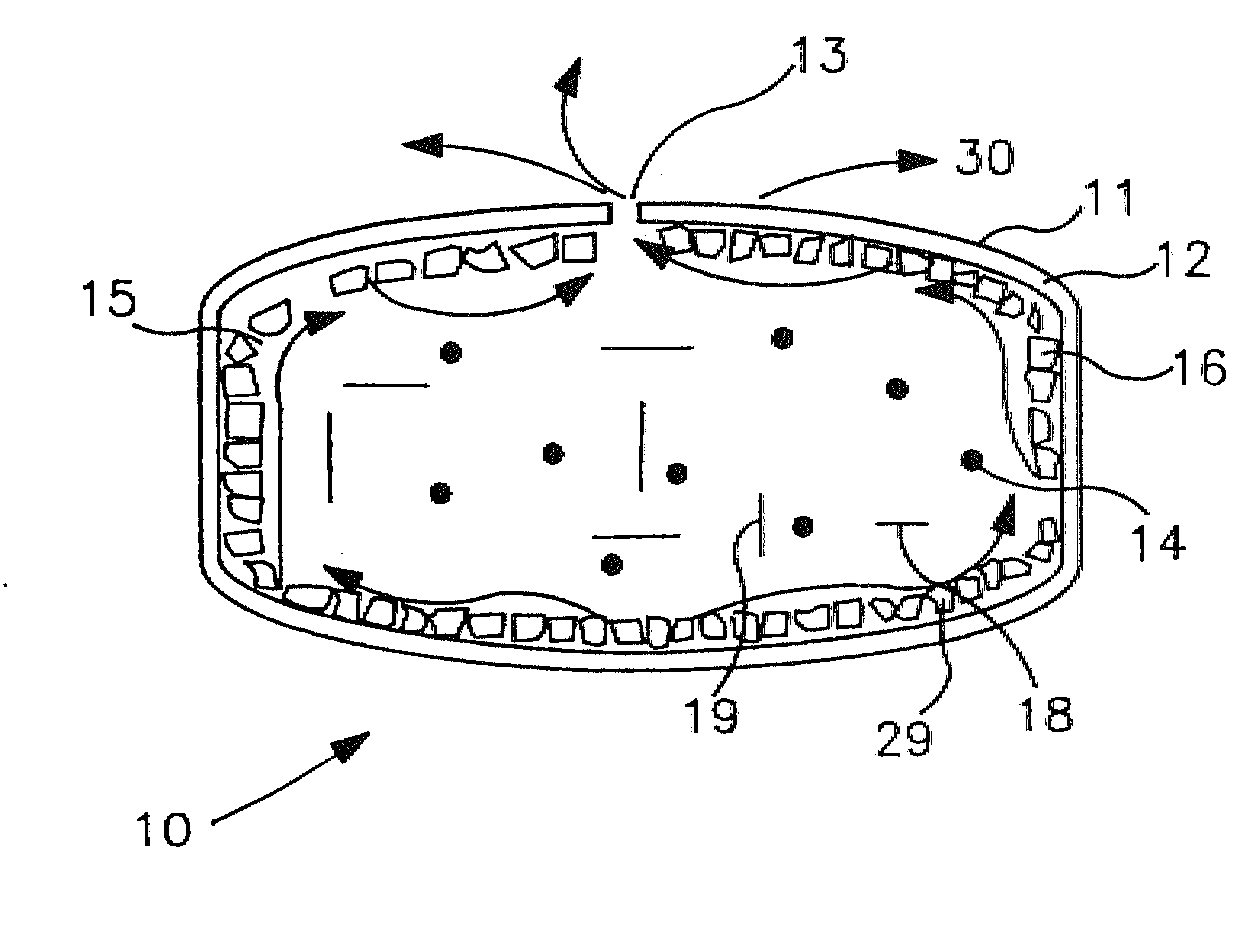
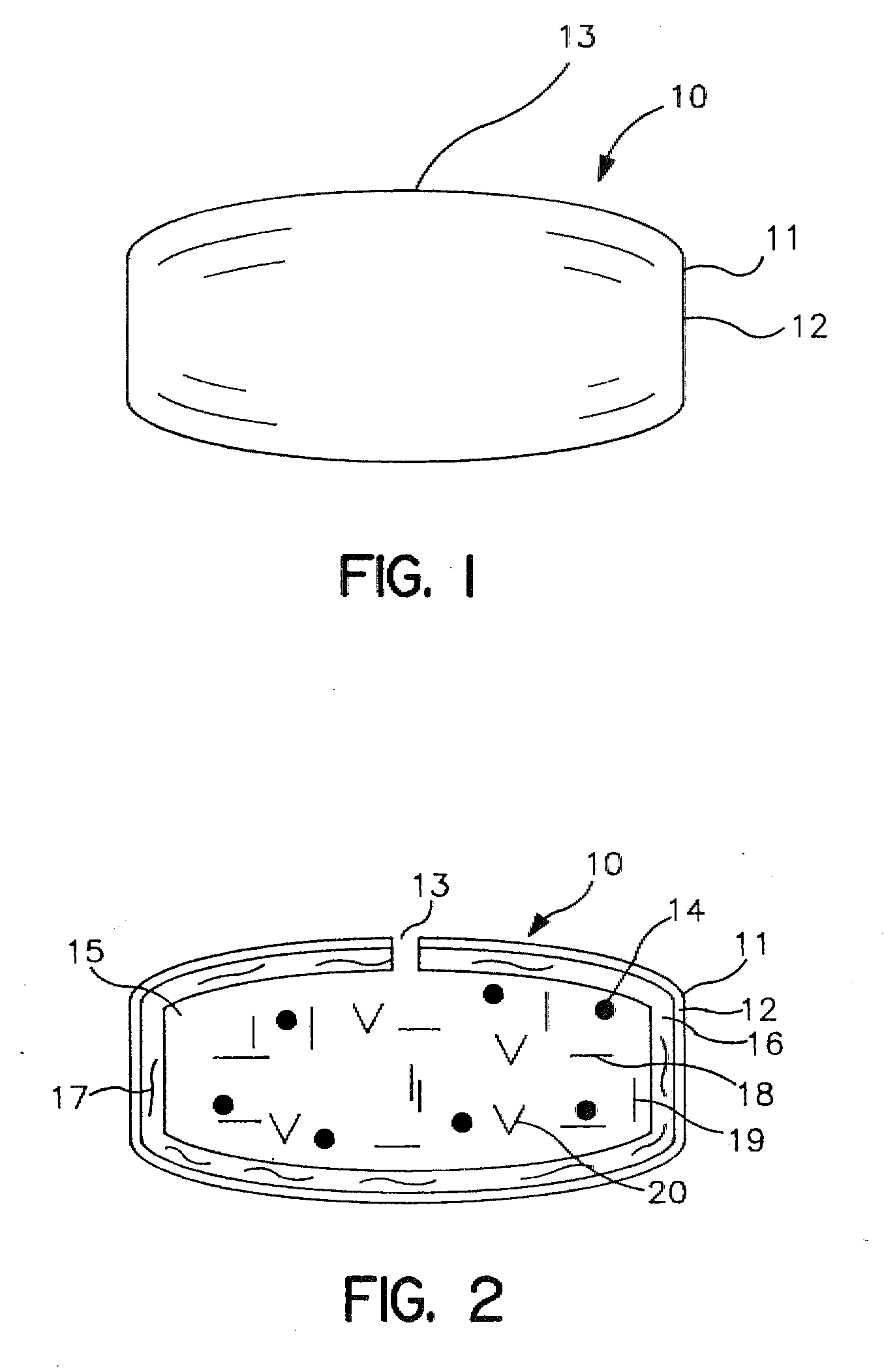
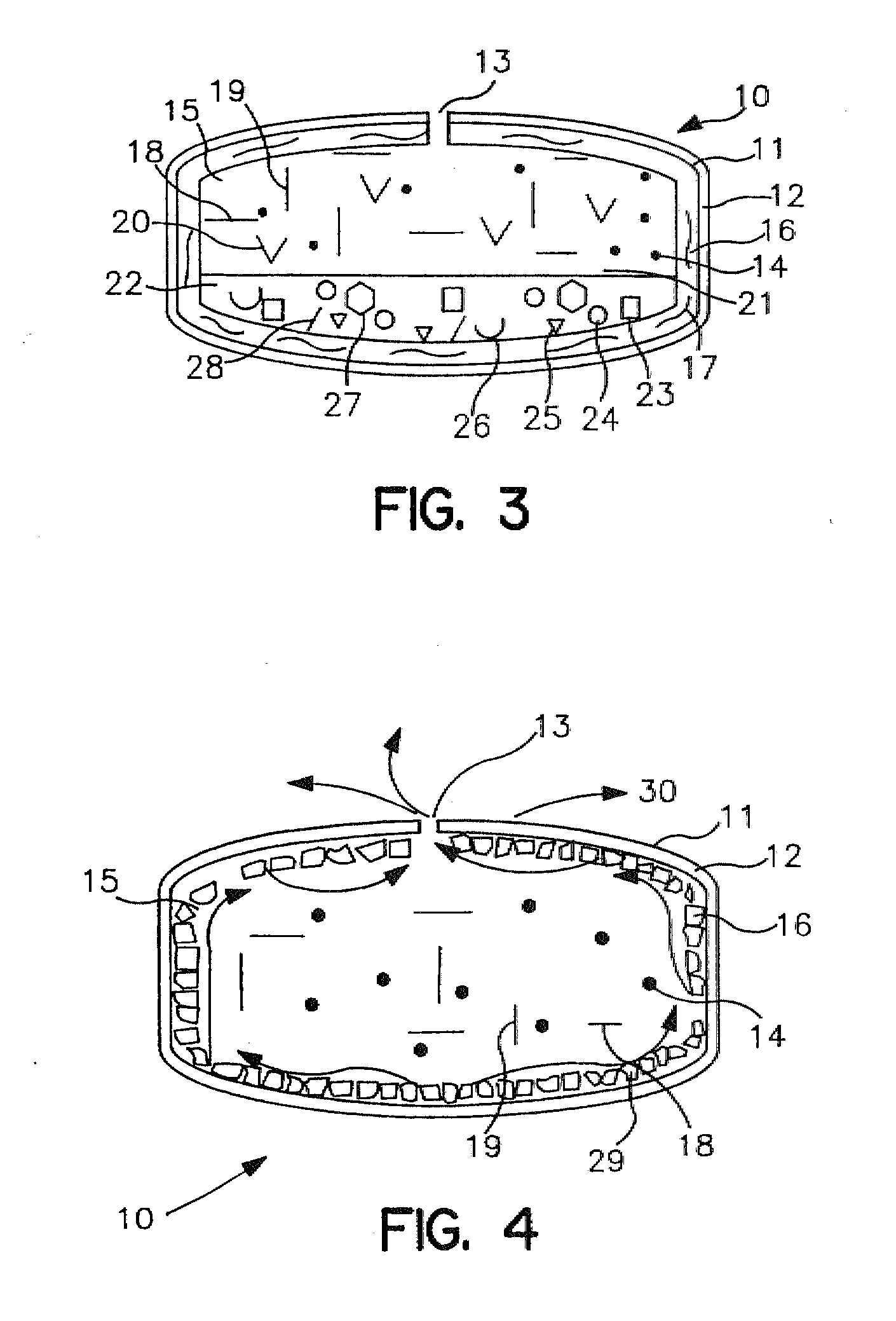
A zero-order release pharmaceutical dosage form for oral administration to a patient comprising a core tablet sheathed in an annular body of compressed powder or granular material is provided. A preferred embodiment of the zero-order release pharmaceutical dosage form is a solid pharmaceutical dosage form which reduces contact of the active ingredient in solid form with the mucosa lining the gastrointestinal tract, which is particularly advantageous for delivering an ulcerative drug. A process for making the zero-order release pharmaceutical dosage form are also provided.
Owner:TEVA PHARMA IND LTD
Controlled released dosage forms
A zero-order release pharmaceutical dosage form for oral administration to a patient comprising a core tablet sheathed in an annular body of compressed powder or granular material is provided. A preferred embodiment of the zero-order release pharmaceutical dosage form is a solid pharmaceutical dosage form which reduces contact of the active ingredient in solid form with the mucosa lining the gastrointestinal tract, which is particularly advantageous for delivering an ulcerative drug. A process for making the zero-order release pharmaceutical dosage form are also provided.
Owner:TEVA PHARMA IND LTD
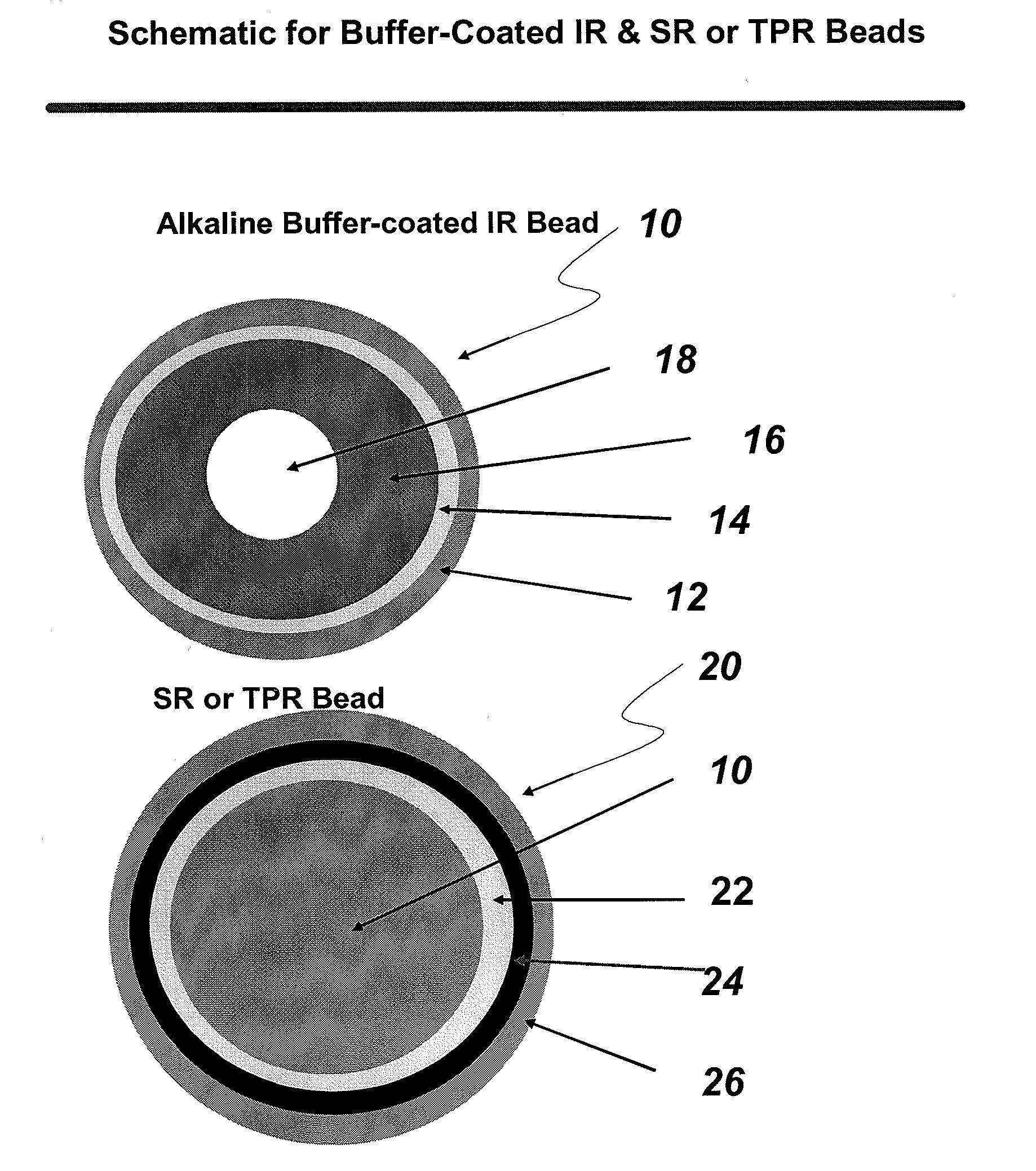
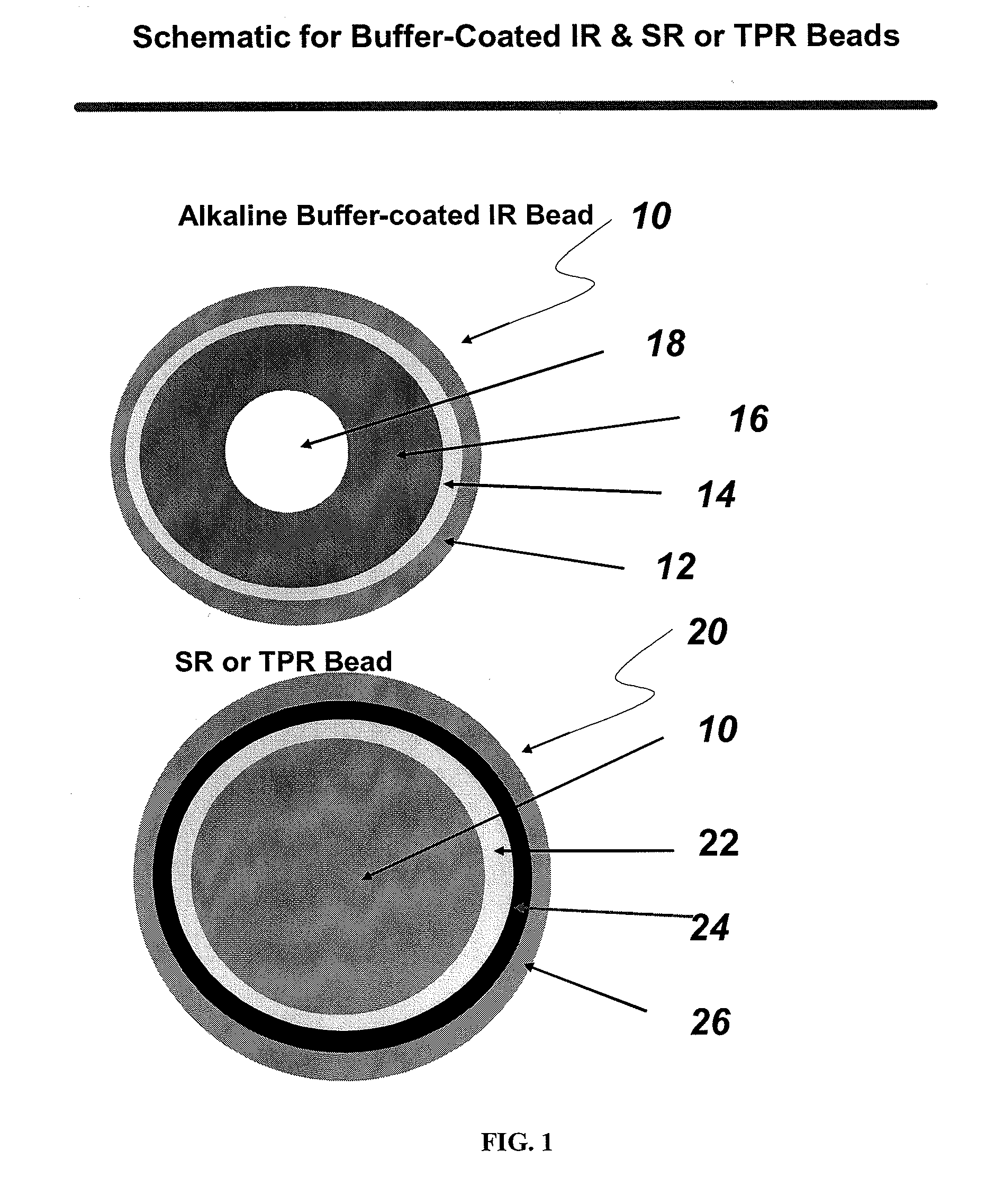
Compositions comprising weakly basic drugs and controlled-release dosage forms
InactiveUS20090258066A1Antibacterial agentsNervous disorderOrally disintegrating tabletMicroparticle
The present invention is directed to pharmaceutical compositions, and methods of making such compositions, comprising microparticles containing a weakly basic drug core, a layer of alkaline buffer, and a controlled-release coating. The present invention is also directed to pharmaceutical dosage forms, including orally disintegrating tablets, conventional tablets, and capsules, and methods for their preparation.
Owner:ADARE PHARM INC
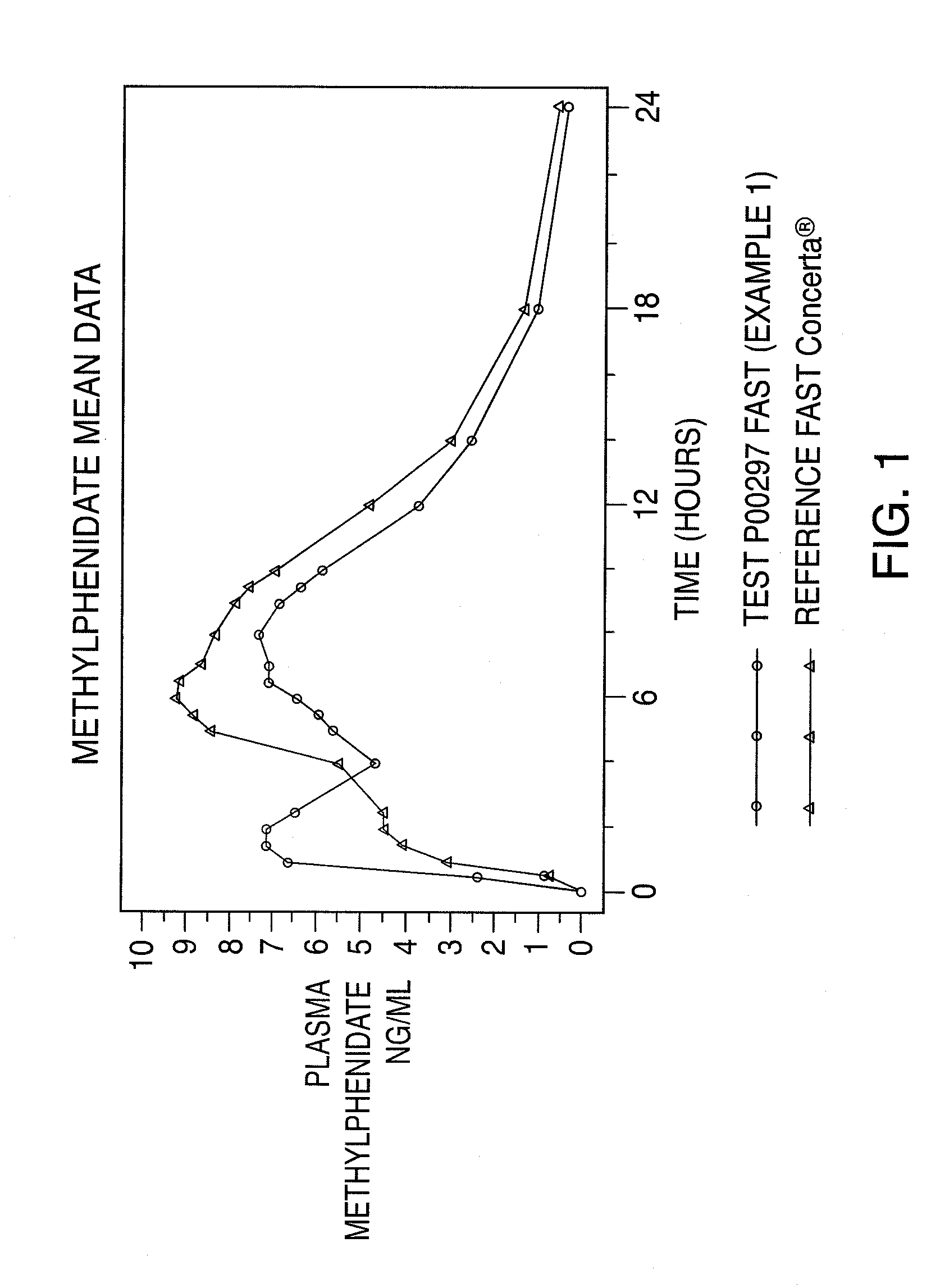
Oral controlled release dosage form
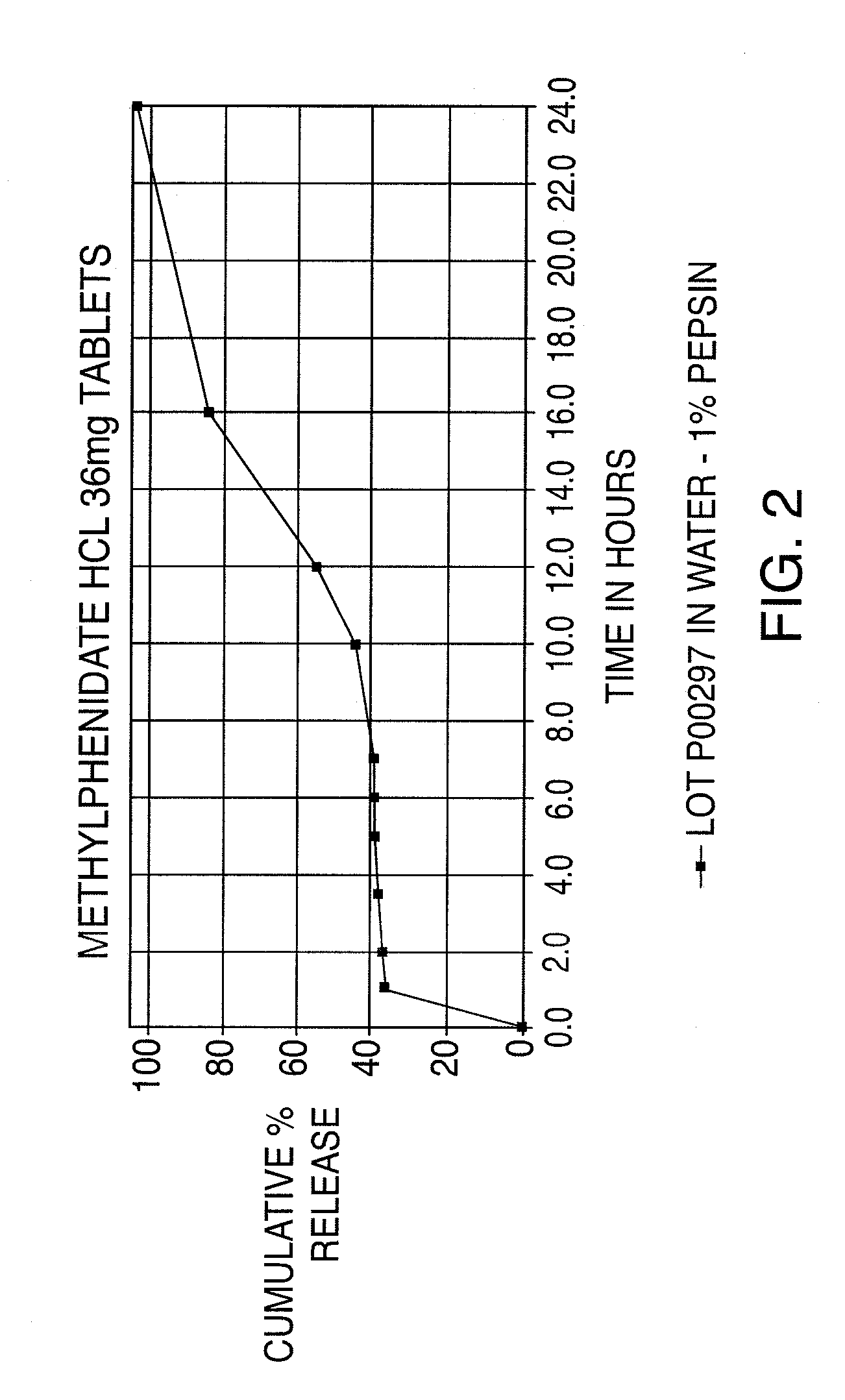
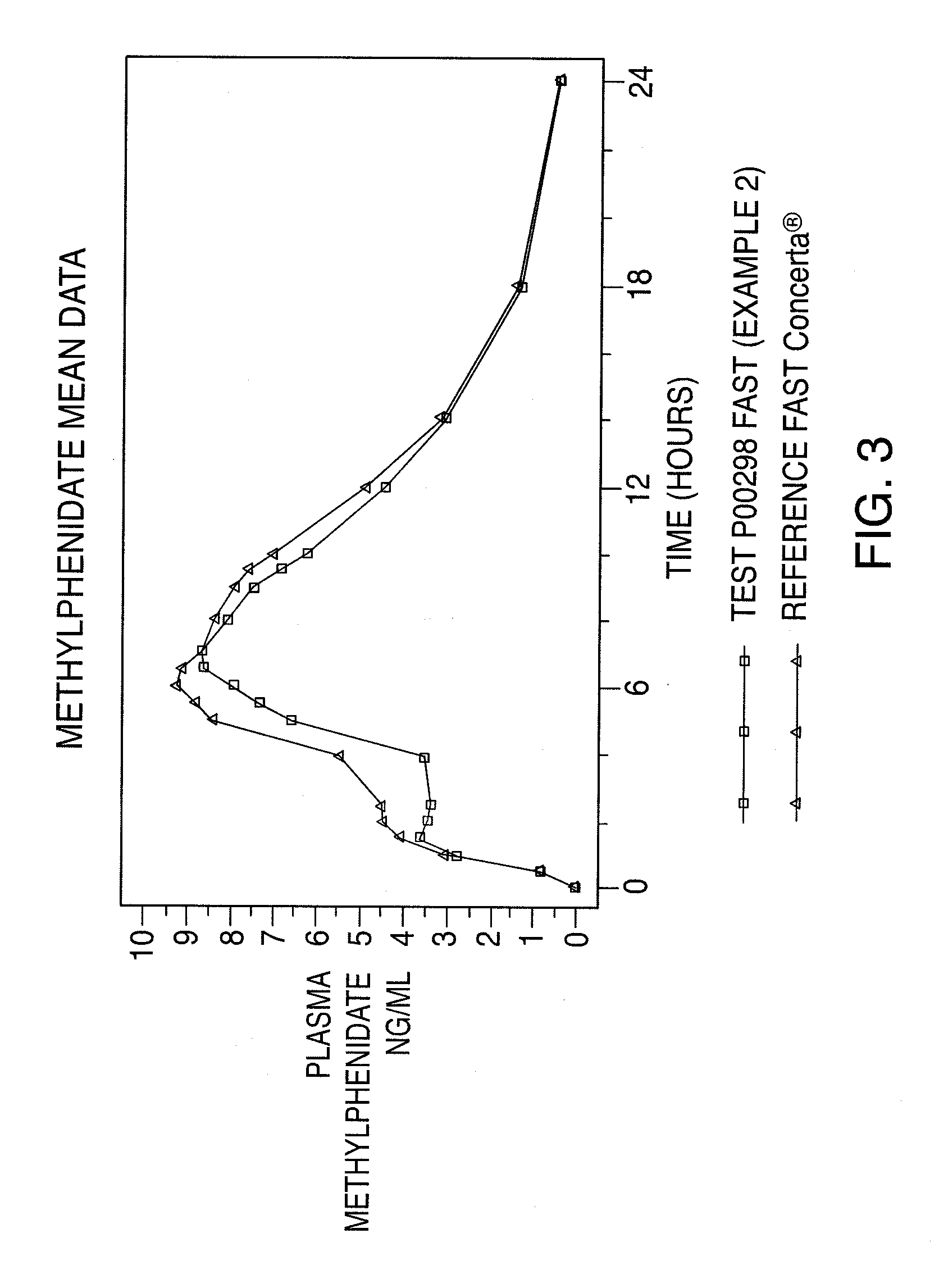
InactiveUS20110268799A1Reproduces effectImprove delivery efficiencyBiocideNervous disorderMethylphenidate HydrochlorideOral medication
A dosage form that provides a controlled release solid dosage form for the oral administration of a central nervous system stimulant, preferably methylphenidate hydrochloride.
Owner:ANDRX PHARMA INC
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20100028421A1BiocidePeptide/protein ingredientsDelayed Release Dosage FormAdditive ingredient
The invention relates to a solid oral dosage form comprising a pharmaceutically active ingredient in combination with an enhancer which enhances the bioavailability and / or the absorption of the active ingredient. Accordingly, a solid oral dosage form comprises a drug and an enhancer wherein the enhancer is a medium chain fatty acid ester, ether or salt or a derivative of a medium chain fatty acid, which is, preferably, solid at room temperature and which has a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
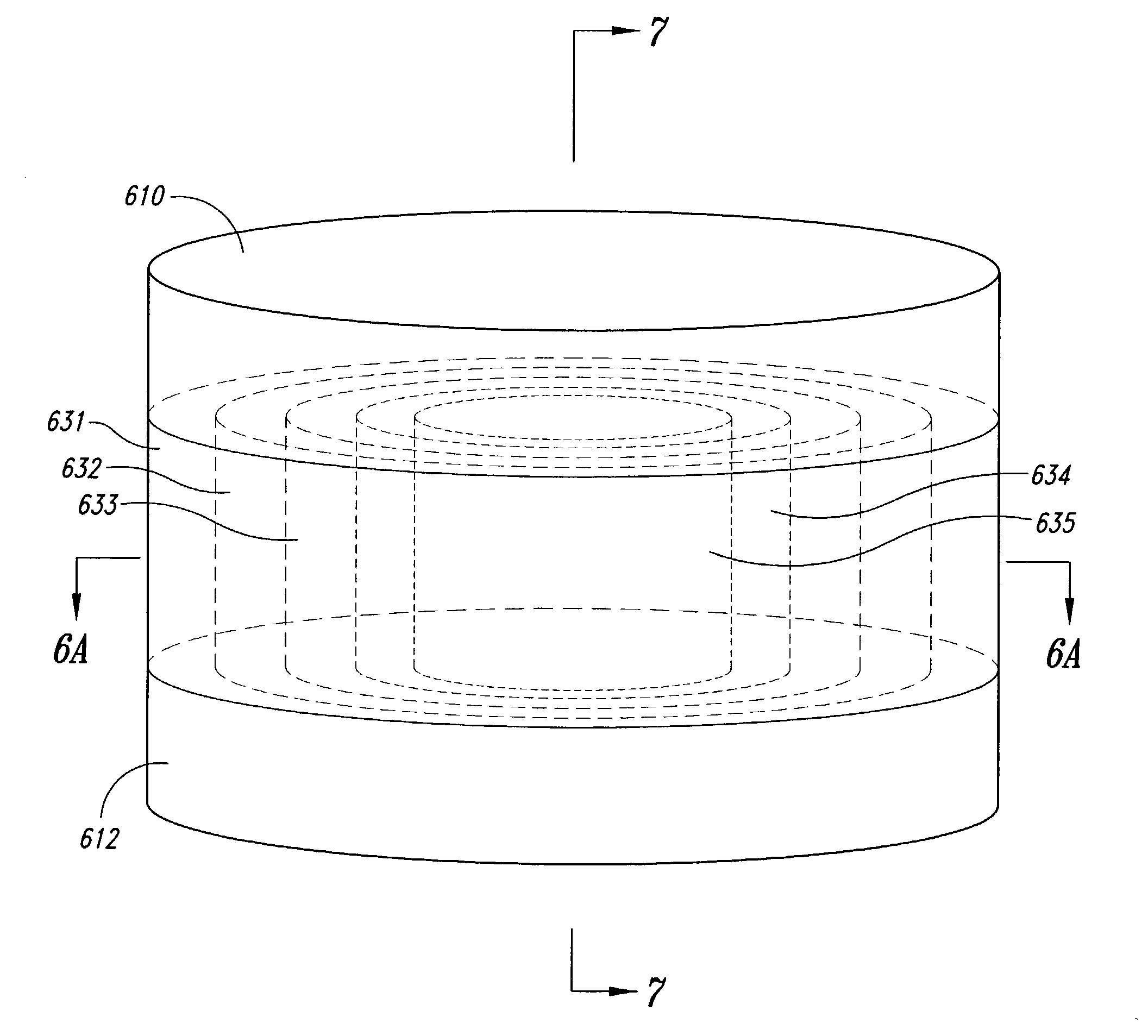
Volume Efficient Controlled Release Dosage Form
InactiveUS20070184112A1Easy loadingOrganic active ingredientsPharmaceutical non-active ingredientsBi layerActive agent
A dosage form that facilitates the controlled release of an active agent at a desired release rate or release rate profile includes a bi-layer membrane system and an osmotic core. The bi-layer membrane system includes a semipermeable membrane and an osmosensitive membrane and forms an internal compartment occupied by the osmotic core. The osmotic core includes an active agent composition and a light push layer. A passageway is formed through the bi-layer membrane system and permits expulsion of the active agent composition from the dosage form during operation. The bi-layer membrane system and the osmotic core are formulated and formed to provide controlled release of the active agent included in the active agent composition, while simultaneously facilitating increased loading of active agent within a dosage form of given dimension and increasing the delivery efficiency of such active agent relative to prior osmotic dosage forms including a push layer.
Owner:ALZA CORP
Dosage form for delivery of multiple drug forms
Disclosed are controlled release dosage forms and related methods including: (a) a micronized or liquid base form of a drug; (b) either a pharmaceutically acceptable salt form of the drug or starting materials that are capable of reacting to form a pharmaceutically acceptable salt form of the drug; (c) an upper gastrointestinal system pharmaceutically acceptable salt form releasing structure; and (d) a colonic system base form releasing structure.
Owner:ALZA CORP
Dosage forms for low solubility and or low dissolution rate free acid pharmaceutical agents
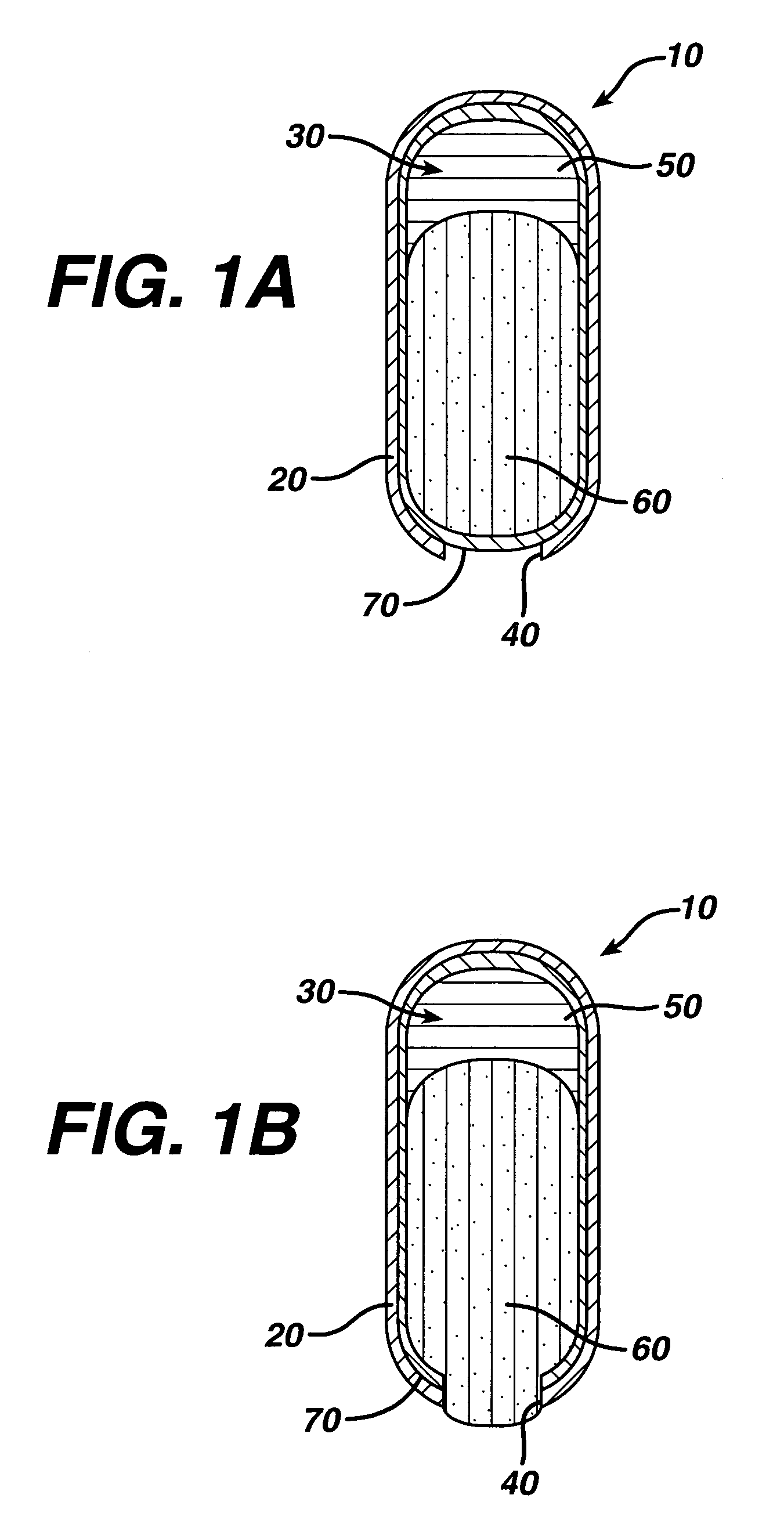
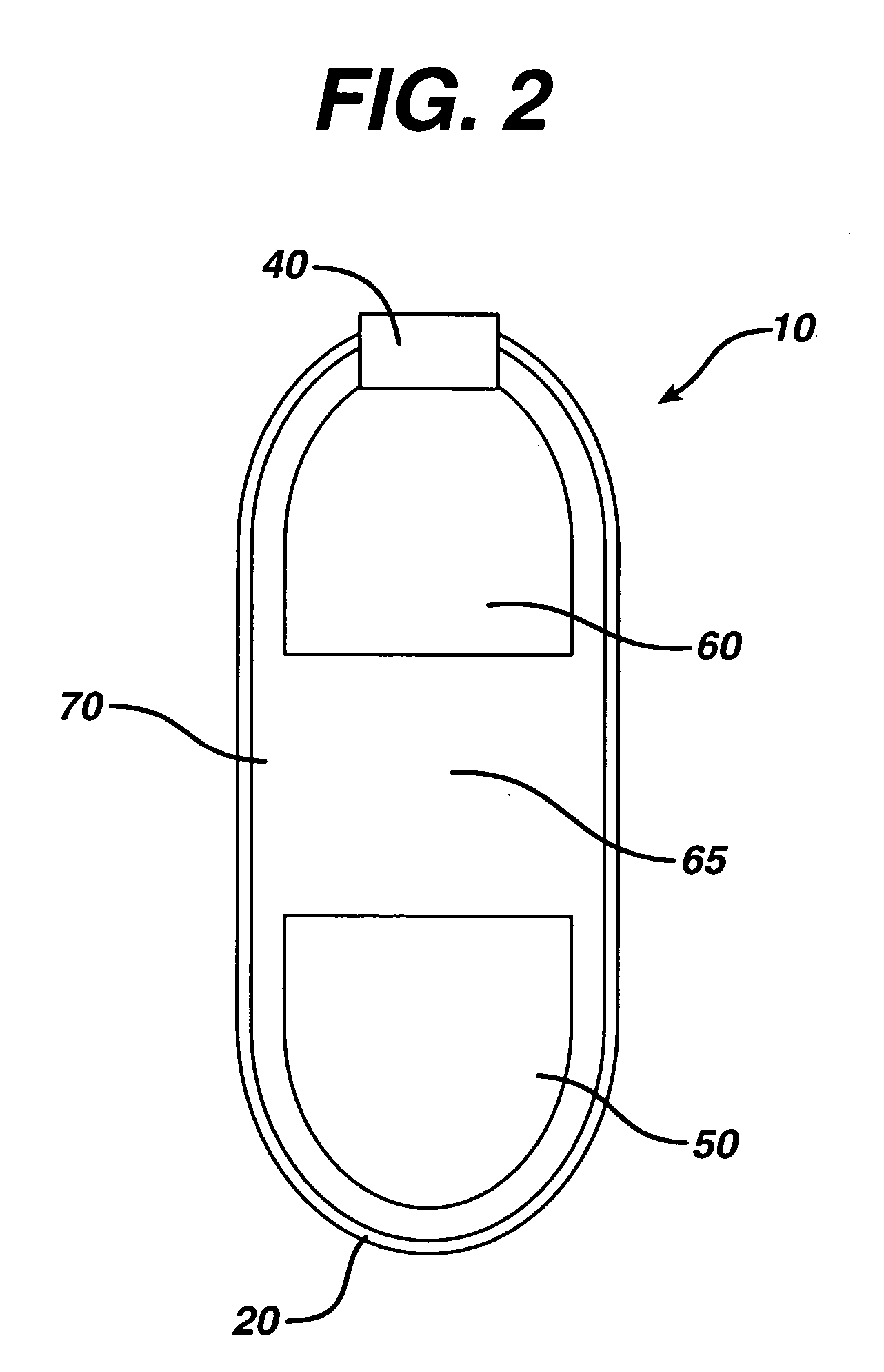
InactiveUS20050287213A1Reduce solubilityLow dissolution ratePill deliveryAnhydride/acid/halide active ingredientsSolubilityTopiramate
An osmotic controlled release dosage form is described comprising a core comprising a first drug composition, wherein the first drug composition comprises topiramate and / or its pharmaceutically acceptable salt; a semi-permeable wall surrounding the core; and an exit orifice through the semi-permeable wall for releasing the first drug composition from the dosage form over a prolonged period of time.
Owner:ALZA CORP
Stepwise delivery of topiramate over prolonged period of time
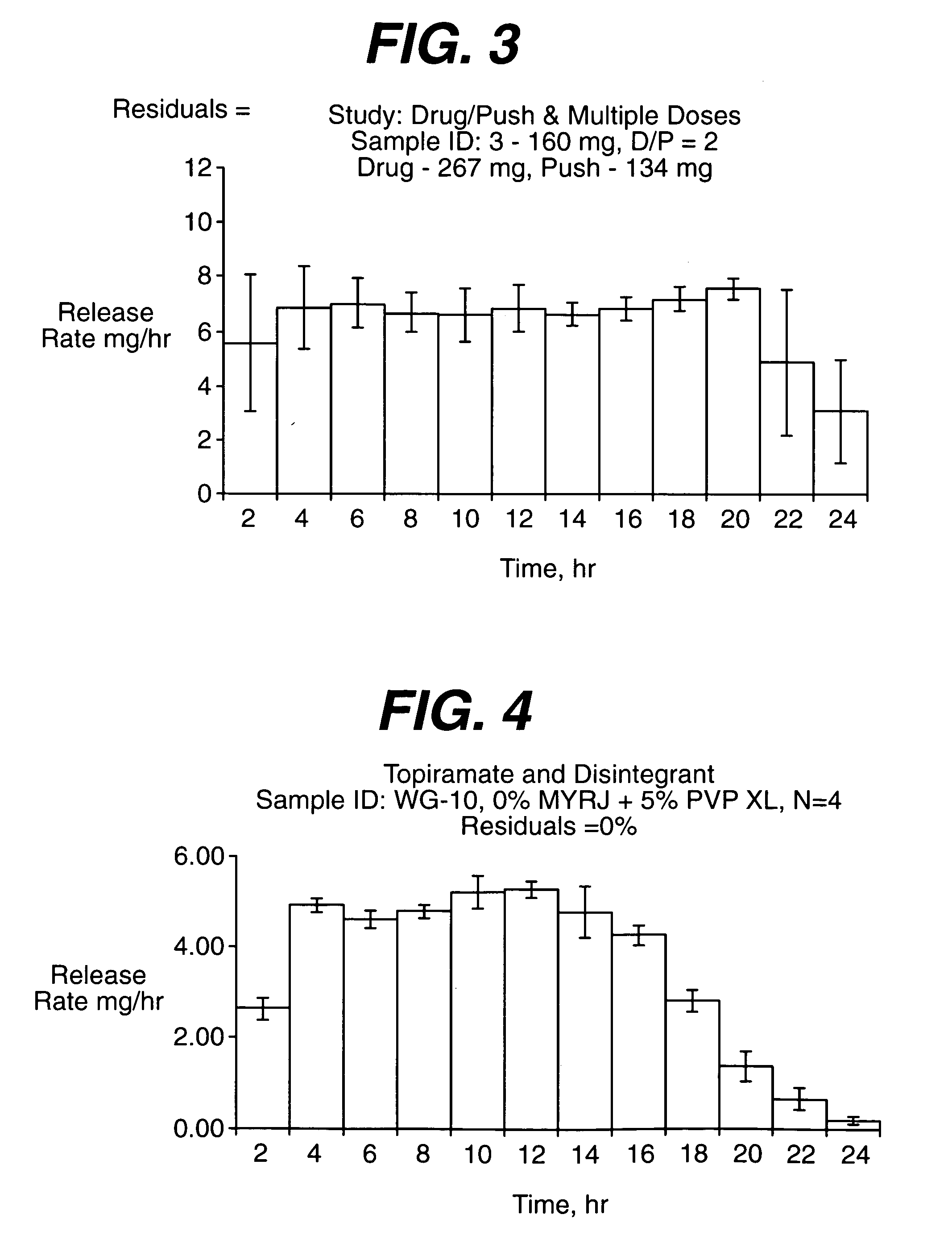
InactiveUS20050136108A1Improve bioavailabilityPromote absorptionNervous disorderOsmotic deliveryTopiramateControlled Release Dosage Form
Compositions and dosage forms for enhanced dispersion of topiramate in a controlled release dosage form released from the dosage form as a dry or substantially dry erodible solid over a prolonged period of time at a stepwise increasing rate of release are described.
Owner:YAM NOYMI V +4
Compositions and methods for inducing satiety and treating non-insulin dependent diabetes mellitus, prediabetic symptoms, insulin resistance and related disease states and conditions
ActiveUS20110268795A1Increase muscle massReduce fatBiocidePeptide/protein ingredientsDiseaseInsulin dependent diabetes
The invention provides methods of treatment that induce satiety in a subject for a period of at least around twenty-four hours by once-daily administration to the subject of a controlled release dosage form, wherein the dosage form is administered while the subject is in the fasted state and at a time of around six to around nine hours prior to the subject's next intended meal, and wherein the dosage form comprises a controlled release composition, which comprises an enterically-coated, ileum hormone-stimulating amount of a nutritional substance and releases the majority of the nutritional substance in vivo upon reaching the subject's ileum. The invention also provides a diagnostic tool for probing the health and disease state of the ileal hormones, excess or deficiencies. The invention provides a safe vehicle for targeted deliveries of chemical, pharmaceuticals, natural substances and nutrition to the ileum. The present invention also provides a method for treating noninsulin dependent diabetes mellitus, pre-diabetic symptoms, and insulin resistance, as well as a number of disease states and conditions including gastrointestinal disorders as otherwise described herein.
Owner:SAPIENZA RES LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com